Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells
- PMID: 23696663
- PMCID: PMC3670366
- DOI: 10.1073/pnas.1220637110
Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells
Abstract
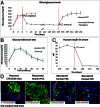
The differentiated state of mature cells of adult organisms is achieved and maintained through the epigenetic regulation of gene expression, which consists of several mechanisms including DNA methylation. The advent of induced pluripotent stem cell technology enabled the conversion of adult cells into any other cell type passing through a stable pluripotency state. However, indefinite pluripotency is unphysiological, inherently labile, and makes cells prone to culture-induced alterations. The direct conversion of one cell type to another without an intermediate pluripotent stage is also possible but, at present, requires the viral transfection of appropriate transcription factors, limiting its therapeutic potential. The aim of this study was to investigate whether it is possible to achieve the direct conversion of an adult cell by exposing it to a demethylating agent immediately followed by differentiating culture conditions. Adult human skin fibroblasts were exposed for 18 h to the DNA methyltransferase inhibitor 5-azacytidine, followed by a three-step protocol for the induction of endocrine pancreatic differentiation that lasted 36 d. At the end of this treatment, 35 ± 8.9% fibroblasts became pancreatic converted cells that acquired an epithelial morphology, produced insulin, and then released the hormone in response to a physiological glucose challenge in vitro. Furthermore, pancreatic converted cells were able to protect recipient mice against streptozotocin-induced diabetes, restoring a physiological response to glucose tolerance tests. This work shows that it is possible to convert adult fibroblasts into insulin-secreting cells, avoiding both a stable pluripotent stage and any transgenic modification.
Keywords: cell plasticity; pancreatic beta cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lengner CJ. iPS cell technology in regenerative medicine. Ann N Y Acad Sci. 2010;1192:38–44. - PubMed
-
- Cohen DE, Melton D. Turning straw into gold: Directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12(4):243–252. - PubMed
-
- Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31(1):36–45. - PubMed
-
- Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22(7):833–840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

