Development of cortical microstructure in the preterm human brain
- PMID: 23696665
- PMCID: PMC3677430
- DOI: 10.1073/pnas.1301652110
Development of cortical microstructure in the preterm human brain
Abstract
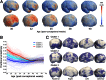
Cortical maturation was studied in 65 infants between 27 and 46 wk postconception using structural and diffusion magnetic resonance imaging. Alterations in neural structure and complexity were inferred from changes in mean diffusivity and fractional anisotropy, analyzed by sampling regions of interest and also by a unique whole-cortex mapping approach. Mean diffusivity was higher in gyri than sulci and in frontal compared with occipital lobes, decreasing consistently throughout the study period. Fractional anisotropy declined until 38 wk, with initial values and rates of change higher in gyri, frontal and temporal poles, and parietal cortex; and lower in sulcal, perirolandic, and medial occipital cortex. Neuroanatomical studies and experimental diffusion-anatomic correlations strongly suggested the interpretation that cellular and synaptic complexity and density increased steadily throughout the period, whereas elongation and branching of dendrites orthogonal to cortical columns was later and faster in higher-order association cortex, proceeding rapidly before becoming undetectable after 38 wk. The rate of microstructural maturation correlated locally with cortical growth, and predicted higher neurodevelopmental test scores at 2 y of age. Cortical microstructural development was reduced in a dose-dependent fashion by longer premature exposure to the extrauterine environment, and preterm infants at term-corrected age possessed less mature cortex than term-born infants. The results are compatible with predictions of the tension theory of cortical growth and show that rapidly developing cortical microstructure is vulnerable to the effects of premature birth, suggesting a mechanism for the adverse effects of preterm delivery on cognitive function.
Keywords: DTI; brain development; preterm birth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–122. - PubMed
-
- Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995;8(7-8):375–386. - PubMed
-
- Neil JJ, et al. Normal brain in human newborns: Apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209(1):57–66. - PubMed
-
- Gupta RK, et al. Diffusion tensor imaging of the developing human cerebrum. J Neurosci Res. 2005;81(2):172–178. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

