High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates
- PMID: 23696746
- PMCID: PMC3656092
- DOI: 10.1371/journal.pgen.1003495
High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates
Abstract
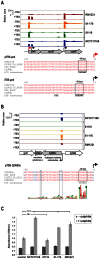
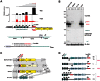
Campylobacter jejuni is currently the leading cause of bacterial gastroenteritis in humans. Comparison of multiple Campylobacter strains revealed a high genetic and phenotypic diversity. However, little is known about differences in transcriptome organization, gene expression, and small RNA (sRNA) repertoires. Here we present the first comparative primary transcriptome analysis based on the differential RNA-seq (dRNA-seq) of four C. jejuni isolates. Our approach includes a novel, generic method for the automated annotation of transcriptional start sites (TSS), which allowed us to provide genome-wide promoter maps in the analyzed strains. These global TSS maps are refined through the integration of a SuperGenome approach that allows for a comparative TSS annotation by mapping RNA-seq data of multiple strains into a common coordinate system derived from a whole-genome alignment. Considering the steadily increasing amount of RNA-seq studies, our automated TSS annotation will not only facilitate transcriptome annotation for a wider range of pro- and eukaryotes but can also be adapted for the analysis among different growth or stress conditions. Our comparative dRNA-seq analysis revealed conservation of most TSS, but also single-nucleotide-polymorphisms (SNP) in promoter regions, which lead to strain-specific transcriptional output. Furthermore, we identified strain-specific sRNA repertoires that could contribute to differential gene regulation among strains. In addition, we identified a novel minimal CRISPR-system in Campylobacter of the type-II CRISPR subtype, which relies on the host factor RNase III and a trans-encoded sRNA for maturation of crRNAs. This minimal system of Campylobacter, which seems active in only some strains, employs a unique maturation pathway, since the crRNAs are transcribed from individual promoters in the upstream repeats and thereby minimize the requirements for the maturation machinery. Overall, our study provides new insights into strain-specific transcriptome organization and sRNAs, and reveals genes that could modulate phenotypic variation among strains despite high conservation at the DNA level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- van Vliet AH (2010) Next generation sequencing of microbial transcriptomes: challenges and opportunities. FEMS Microbiol Lett 302: 1–7. - PubMed
-
- Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, et al. (2010) The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464: 250–255. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

