Zinc transporter 8 and MAP3865c homologous epitopes are recognized at T1D onset in Sardinian children
- PMID: 23696819
- PMCID: PMC3656963
- DOI: 10.1371/journal.pone.0063371
Zinc transporter 8 and MAP3865c homologous epitopes are recognized at T1D onset in Sardinian children
Abstract
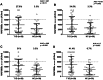
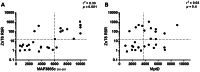
Our group has recently demonstrated that Mycobacterium avium subspecies paratuberculosis (MAP) infection significantly associates with T1D in Sardinian adult patients. Due to the potential role played by MAP in T1D pathogenesis, it is relevant to better characterize the prevalence of anti-MAP antibodies (Abs) in the Sardinian population, studying newly diagnosed T1D children. Therefore, we investigated the seroreactivity against epitopes derived from the ZnT8 autoantigen involved in children at T1D onset and their homologous sequences of the MAP3865c protein. Moreover, sera from all individuals were tested for the presence of Abs against: the corresponding ZnT8 C-terminal region, the MAP specific protein MptD, the T1D autoantigen GAD65 and the T1D unrelated Acetylcholine Receptor. The novel MAP3865c281-287 epitope emerges here as the major C-terminal epitope recognized. Intriguingly ZnT8186-194 immunodominant peptide was cross-reactive with the homologous sequences MAP3865c133-141, strengthening the hypothesis that MAP could be an environmental trigger of T1D through a molecular mimicry mechanism. All eight epitopes were recognized by circulating Abs in T1D children in comparison to healthy controls, suggesting that these Abs could be biomarkers of T1D. It would be relevant to investigate larger cohorts of children, followed over time, to elucidate whether Ab titers against these MAP/Znt8 epitopes wane after diagnosis.
Conflict of interest statement
Figures

References
-
- Sechi LA, Paccagnini D, Salza S, Pacifico A, Ahmed N, et al. (2008) Mycobacterium avium subspecies paratuberculosis bacteremia in type 1 diabetes mellitus: an infectious trigger? Clin Infect Dis 46: 148–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

