Transient and stable expression of the neurotensin receptor NTS1: a comparison of the baculovirus-insect cell and the T-REx-293 expression systems
- PMID: 23696845
- PMCID: PMC3656039
- DOI: 10.1371/journal.pone.0063679
Transient and stable expression of the neurotensin receptor NTS1: a comparison of the baculovirus-insect cell and the T-REx-293 expression systems
Abstract
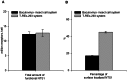
Nowadays, baculovirus-infected insect cells and tetracycline-inducible mammalian cell lines (T-REx-293) are intensively used for G protein-coupled receptor (GPCR) production for crystallography purposes. Here we constructed a suspension T-REx-293 cell line to stably express an engineered neurotensin receptor 1 (NTS1) mutant and we quantitatively compared this cell line with the transient baculovirus-insect cell system throughout a milligram-scale NTS1 expression and purification process. The two systems were comparable with respect to functional NTS1 expression levels and receptor binding affinity for the agonist [(3)H] neurotensin. However, NTS1 surface display on T-REx-293 cells determined by radio-ligand binding assays was 2.8 fold higher than that on insect cells. This work demonstrates two approaches for preparing milligram quantities of purified NTS1 suitable for structural studies and provides useful input to users in choosing and optimizing an appropriate expression host for other GPCRs.
Conflict of interest statement
Figures




References
-
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650. - PubMed
-
- Grisshammer R, Tate CG (1995) Overexpression of integral membrane proteins for structural studies. Q Rev Biophys 28: 315–422. - PubMed
-
- Tate CG, Grisshammer R (1996) Heterologous expression of G-protein-coupled receptors. Trends Biotechnol 14: 426–430. - PubMed
-
- Carraway R, Leeman SE (1973) The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 248: 6854–6861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

