Stochastic model of Tsc1 lesions in mouse brain
- PMID: 23696872
- PMCID: PMC3655945
- DOI: 10.1371/journal.pone.0064224
Stochastic model of Tsc1 lesions in mouse brain
Erratum in
- PLoS One. 2013;8(11). doi:10.1371/annotation/6a5b0a50-27e4-49bc-b82a-9267dd63af53. Zuang, Xuan [corrected to Zhang, Xuan]
Abstract
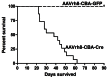
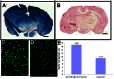
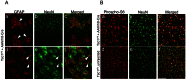
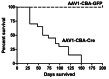
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder due to mutations in either TSC1 or TSC2 that affects many organs with hamartomas and tumors. TSC-associated brain lesions include subependymal nodules, subependymal giant cell astrocytomas and tubers. Neurologic manifestations in TSC comprise a high frequency of mental retardation and developmental disorders including autism, as well as epilepsy. Here, we describe a new mouse model of TSC brain lesions in which complete loss of Tsc1 is achieved in multiple brain cell types in a stochastic pattern. Injection of an adeno-associated virus vector encoding Cre recombinase into the cerebral ventricles of mice homozygous for a Tsc1 conditional allele on the day of birth led to reduced survival, and pathologic findings of enlarged neurons, cortical heterotopias, subependymal nodules, and hydrocephalus. The severity of clinical and pathologic findings as well as survival was shown to be dependent upon the dose and serotype of Cre virus injected. Although several other models of TSC brain disease exist, this model is unique in that the pathology reflects a variety of TSC-associated lesions involving different numbers and types of cells. This model provides a valuable and unique addition for therapeutic assessment.
Conflict of interest statement
Figures

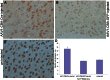
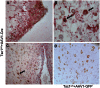

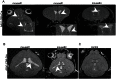



References
-
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, et al. (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277: 805–808. - PubMed
-
- European Chromosome 16 Tuberous Sclerosis C (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75: 1305–1315. - PubMed
-
- Kwiatkowski DJ, Whittemore VH, Thiele EA (2010) Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim, Germany: Wiley-Blackwell.
-
- Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. New Eng J Med 355: 1345–1356. - PubMed
-
- Bordarier C, Lellouch-Tubiana A, Robain O (1994) Cardiac rhabdomyoma and tuberous sclerosis in three fetuses: a neuropathological study. Brain Dev 16: 467–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

