Elevated inflammatory mediators in adults with oculorespiratory syndrome following influenza immunization: a public health agency of Canada/Canadian Institutes of Health Research Influenza Research Network Study
- PMID: 23697573
- PMCID: PMC3754507
- DOI: 10.1128/CVI.00659-12
Elevated inflammatory mediators in adults with oculorespiratory syndrome following influenza immunization: a public health agency of Canada/Canadian Institutes of Health Research Influenza Research Network Study
Abstract
Oculorespiratory syndrome (ORS) is an infrequent adverse event following influenza vaccination. Its clinical presentation suggests that ORS is an immune-mediated phenomenon, but studies of symptomatic individuals have been few. This study measured cytokine levels in peripheral blood samples following influenza vaccination in those with and without current ORS symptoms. Canadian adults receiving the 2010-2011 seasonal influenza vaccine were recruited and asked to promptly report any adverse effects. ORS symptoms occurring 4 to 48 h after vaccination were identified using previously published criteria. Two blood samples were collected from each subject to measure blood plasma cytokine and hemagglutination inhibition antibody (HAI) titers; visit 1 occurred during the acute disease phase or 4 to 72 h after vaccination for controls, and visit 2 occurred another 21 days postimmunization. Nine ORS cases and 35 controls were enrolled. The median age of ORS cases was 49 years, and 89% were female. Most cases had multiple symptoms, but none required medical care. HAI titers before and after vaccination were similar for the cases and controls. Blood plasma cytokine concentrations did not differ between the ORS cases and controls for most cytokines measured (interleukin 4 [IL-4], IL-5, IL-10, IL-13, IL-1α, IL-8, tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], and IL-17A). However, ORS cases had higher levels of IL-10 and IL-3 than the controls at visits 1 and 2, even after all symptoms had subsided. Persistent higher levels of IL-10 and IL-3 in ORS cases suggest that host factors may have predisposed these individuals to develop ORS following influenza vaccination. Further investigations are warranted, as they might identify subjects who are at risk for ORS prior to vaccination.
Figures
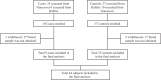
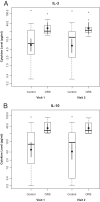
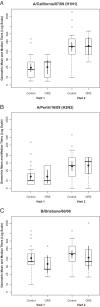
References
-
- Skowronski DM, Strauss B, De Serres G, MacDonald D, Marion SA, Naus M, Patrick DM, Kendall P. 2003. Oculo-respiratory syndrome: a new influenza vaccine-associated adverse event? Clin. Infect. Dis. 36:705–713 - PubMed
-
- Public Health Agency of Canada 2001. An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Statement on influenza vaccination for the 2001-2002 season. Can. Commun. Dis. Rep. 27:1–24 - PubMed
-
- De Serres G, Boulianne N, Duval B, Rochette L, Grenier JL, Roussel R, Donaldson D, Tremblay M, Toth E, Ménard S, Landry M, Robert Y. 2003. Oculo-respiratory syndrome following influenza vaccination: evidence for occurrence with more than one influenza vaccine. Vaccine 21:2346–2353 - PubMed
-
- Skowronski DM, De Serres G, Hebert J, Stark D, Warrington R, Macnabb J, Shadmani R, Rochette L, MacDonald D, Patrick DM, Duval B. 2002. Skin testing to evaluate oculo-respiratory syndrome (ORS) associated with influenza vaccination during the 2000-2001 season. Vaccine 20:2713–2719 - PubMed
-
- Spellberg B, Edwards JE., Jr 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76–102 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

