Comparative effectiveness research in oncology
- PMID: 23697601
- PMCID: PMC4063403
- DOI: 10.1634/theoncologist.2012-0445
Comparative effectiveness research in oncology
Abstract
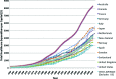
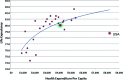
Although randomized controlled trials represent the gold standard for comparative effective research (CER), a number of additional methods are available when randomized controlled trials are lacking or inconclusive because of the limitations of such trials. In addition to more relevant, efficient, and generalizable trials, there is a need for additional approaches utilizing rigorous methodology while fully recognizing their inherent limitations. CER is an important construct for defining and summarizing evidence on effectiveness and safety and comparing the value of competing strategies so that patients, providers, and policymakers can be offered appropriate recommendations for optimal patient care. Nevertheless, methodological as well as political and social challenges for CER remain. CER requires constant and sophisticated methodological oversight of study design and analysis similar to that required for randomized trials to reduce the potential for bias. At the same time, if appropriately conducted, CER offers an opportunity to identify the most effective and safe approach to patient care. Despite rising and unsustainable increases in health care costs, an even greater challenge to the implementation of CER arises from the social and political environment questioning the very motives and goals of CER. Oncologists and oncology professional societies are uniquely positioned to provide informed clinical and methodological expertise to steer the appropriate application of CER toward critical discussions related to health care costs, cost-effectiveness, and the comparative value of the available options for appropriate care of patients with cancer.
Keywords: Clinical trials; Comparative effectiveness; Cost-effectiveness; Health policy; Outcomes.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Comment in
-
Preparing for success with comparative effectiveness research.Oncologist. 2013 Jun;18(6):655-7. doi: 10.1634/theoncologist.2013-0213. Oncologist. 2013. PMID: 23814162 Free PMC article.
References
-
- Davis K. Slowing the growth of health care costs—learning from international experience. N Engl J Med. 2008;359:1751–1755. - PubMed
-
- Oberlander J. Unfinished journey—a century of health care reform in the United States. N Engl J Med. 2012;367:585–590. - PubMed
-
- Lyman GH. Comparative effectiveness research in oncology: The need for clarity, transparency and vision. Cancer Invest. 2009;27:593–597. - PubMed
-
- Goodman SN. Quasi-random reflections on randomized controlled trials and comparative effectiveness research. Clin Trials. 2012;9:22–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

