Usefulness of serum carcinoembryonic antigen (CEA) in evaluating response to chemotherapy in patients with advanced non small-cell lung cancer: a prospective cohort study
- PMID: 23697613
- PMCID: PMC3665670
- DOI: 10.1186/1471-2407-13-254
Usefulness of serum carcinoembryonic antigen (CEA) in evaluating response to chemotherapy in patients with advanced non small-cell lung cancer: a prospective cohort study
Abstract
Background: High serum carcinoembryonic antigen (CEA) levels are an independent prognostic factor for recurrence and survival in patients with non-small cell lung cancer (NSCLC). Its role as a predictive marker of treatment response has not been widely characterized.
Methods: 180 patients with advanced NSCLC (stage IIIB or Stage IV), who had an elevated CEA serum level (>10 ng/ml) at baseline and who had no more than one previous chemotherapy regimen, were included. CEA levels were measured after two treatment cycles of platinum based chemotherapy (93%) or a tyrosine kinase inhibitor (7%). We evaluate the change in serum CEA levels and the association with response measured by RECIST criteria.
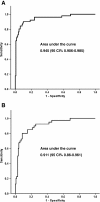
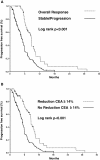
Results: After two chemotherapy cycles, the patients who achieved an objective response (OR, 28.3%) had a reduction of CEA levels of 55.6% (95%CI [box drawings light horizontal]64.3 to [box drawings light horizontal]46.8) compared to its basal level, with an area under the ROC curve (AURC) of 0.945 (95%CI 0.91-0.99), and a sensitivity and specificity of 90.2 and 89.9%, respectively, for a CEA reduction of ≥14%. Patients that achieved a decrease in CEA levels ≥14% presented an overall response in 78% of cases, stable disease in 20.3% and progression in 1.7%, while patients that did not attain a reduction ≥14% had an overall response of 4.1%, stable disease of 63.6% and progression of 32.2% (p < 0.001). Patients with stable (49.4%) and progressive disease (22.2%) had an increase of CEA levels of 9.4% (95%CI 1.5-17.3) and 87.5% (95%CI 60.9-114) from baseline, respectively (p < 0.001). The AURC for progressive disease was 0.911 (95%CI 0.86-0.961), with sensitivity and specificity of 85 and 15%, respectively, for a CEA increase of ≥18%. PFS was longer in patients with a ≥14% reduction in CEA (8.7 vs. 5.1 months, p < 0.001). Neither reduction of CEA nor OR were predictive of OS.
Conclusions: A CEA level reduction is a sensitive and specific marker of OR, as well as a sensitive indicator for progression to chemotherapy in patients with advanced NSCLC who had an elevated CEA at baseline and had received no more than one chemotherapy regimen. A 14% decrease in CEA levels is associated with a better PFS.
Figures
Similar articles
-
The role of serum carcinoembryonic antigen in predicting responses to chemotherapy and survival in patients with non-small cell lung cancer.J Cancer Res Ther. 2014 Apr-Jun;10(2):239-43. doi: 10.4103/0973-1482.136541. J Cancer Res Ther. 2014. PMID: 25022372
-
The Role of Change Rates of CYFRA21-1 and CEA in Predicting Chemotherapy Efficacy for Non-Small-Cell Lung Cancer.Comput Math Methods Med. 2021 Sep 21;2021:1951364. doi: 10.1155/2021/1951364. eCollection 2021. Comput Math Methods Med. 2021. Retraction in: Comput Math Methods Med. 2023 Oct 18;2023:9786104. doi: 10.1155/2023/9786104. PMID: 34603482 Free PMC article. Retracted.
-
Association between serum biomarkers CEA and LDH and response in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy.Thorac Cancer. 2020 Jul;11(7):1790-1800. doi: 10.1111/1759-7714.13449. Epub 2020 May 7. Thorac Cancer. 2020. PMID: 32383328 Free PMC article.
-
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer.Lung Cancer. 2012 May;76(2):138-43. doi: 10.1016/j.lungcan.2011.11.012. Epub 2011 Dec 6. Lung Cancer. 2012. PMID: 22153832 Review.
-
Elevated preoperative CEA is associated with subclinical nodal involvement and worse survival in stage I non-small cell lung cancer: a systematic review and meta-analysis.J Cardiothorac Surg. 2020 Oct 15;15(1):318. doi: 10.1186/s13019-020-01353-2. J Cardiothorac Surg. 2020. PMID: 33059696 Free PMC article.
Cited by
-
Machine learning-featured Secretogranin V is a circulating diagnostic biomarker for pancreatic adenocarcinomas associated with adipopenia.Front Oncol. 2022 Aug 17;12:942774. doi: 10.3389/fonc.2022.942774. eCollection 2022. Front Oncol. 2022. PMID: 36059698 Free PMC article.
-
Evaluation of VEGF-C and tumor markers in bronchoalveolar lavage fluid for lung cancer diagnosis.Sci Rep. 2013 Dec 11;3:3473. doi: 10.1038/srep03473. Sci Rep. 2013. PMID: 24326979 Free PMC article.
-
Serum Carcinoembryonic Antigen Levels and the Risk of Whole-body Metastatic Potential in Advanced Non-small Cell Lung Cancer.J Cancer. 2014 Sep 5;5(8):663-9. doi: 10.7150/jca.9871. eCollection 2014. J Cancer. 2014. PMID: 25258647 Free PMC article.
-
Cytokine and epigenetic regulation of CEACAM6 mediates EGFR-driven signaling and drug response in lung adenocarcinoma.NPJ Precis Oncol. 2025 Apr 22;9(1):115. doi: 10.1038/s41698-025-00910-z. NPJ Precis Oncol. 2025. PMID: 40263546 Free PMC article.
-
Response of an HER2-Mutated NSCLC Patient to Trastuzumab Deruxtecan and Monitoring of Plasma ctDNA Levels by Liquid Biopsy.Curr Oncol. 2023 Jan 30;30(2):1692-1698. doi: 10.3390/curroncol30020130. Curr Oncol. 2023. PMID: 36826091 Free PMC article.
References
-
- Paesmans M, Sculier JP, Libert P. Response to chemotherapy has predictive value for further survival of patients with advanced non-small cell lung cancer: 10 years experience of the European Lung Cancer Working Party. Eur J Cancer. 1997;33(14):2326–2332. doi: 10.1016/S0959-8049(97)00325-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

