Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae
- PMID: 23697887
- PMCID: PMC3673611
- DOI: 10.1089/zeb.2012.0821
Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae
Abstract
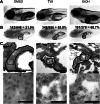
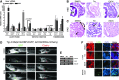
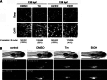
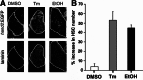
Fatty liver disease in humans can progress from steatosis to hepatocellular injury, fibrosis, cirrhosis, and liver failure. We developed a series of straightforward assays to determine whether zebrafish larvae with either tunicamycin- or ethanol-induced steatosis develop hepatic dysfunction. We found altered expression of genes involved in acute phase response and hepatic function, and impaired hepatocyte secretion and disruption of canaliculi in both models, but glycogen deficiency in hepatocytes and dilation of hepatic vasculature occurred only in ethanol-treated larvae. Hepatic stellate cells (HSCs) become activated during liver injury and HSC numbers increased in both models. Whether the excess lipids in hepatocytes are a direct cause of hepatocyte dysfunction in fatty liver disease has not been defined. We prevented ethanol-induced steatosis by blocking activation of the sterol response element binding proteins (Srebps) using gonzo(mbtps1) mutants and scap morphants and found that hepatocyte dysfunction persisted even in the absence of lipid accumulation. This suggests that lipotoxicity is not the primary cause of hepatic injury in these models of fatty liver disease. This study provides a panel of parameters to assess liver disease that can be easily applied to zebrafish mutants, transgenics, and for drug screening in which liver function is an important consideration.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P30DK026743/DK/NIDDK NIH HHS/United States
- 5R24 CA095823-04/CA/NCI NIH HHS/United States
- 1F32AA021024-01/AA/NIAAA NIH HHS/United States
- T32 CA088796/CA/NCI NIH HHS/United States
- T32 DK007792/DK/NIDDK NIH HHS/United States
- R00 AA020514/AA/NIAAA NIH HHS/United States
- P20AA017067/AA/NIAAA NIH HHS/United States
- K05 AA018408/AA/NIAAA NIH HHS/United States
- 1 S10 RR0 9145-01/RR/NCRR NIH HHS/United States
- P20 AA017067/AA/NIAAA NIH HHS/United States
- K99AA020514/AA/NIAAA NIH HHS/United States
- 5T32CA078207/CA/NCI NIH HHS/United States
- R01 AA018886/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

