"Head-to-side-chain" cyclodepsipeptides of marine origin
- PMID: 23697952
- PMCID: PMC3707169
- DOI: 10.3390/md11051693
"Head-to-side-chain" cyclodepsipeptides of marine origin
Abstract
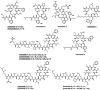
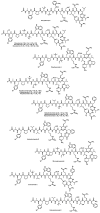
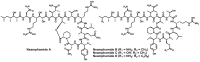
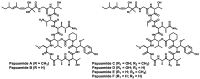
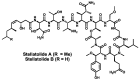
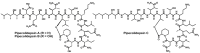
Since the late 1980s, a large number of depsipeptides that contain a new topography, referred to as "head-to-side-chain" cyclodepsipeptides, have been isolated and characterized. These peptides present a unique structural arrangement that comprises a macrocyclic region closed through an ester bond between the C-terminus and a β-hydroxyl group, and terminated with a polyketide moiety or a more simple branched aliphatic acid. This structural pattern, the presence of unique and complex residues, and relevant bioactivity are the main features shared by all the members of this new class of depsipeptides, which are reviewed herein.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

