Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs
- PMID: 23698296
- PMCID: PMC3700183
- DOI: 10.1128/JVI.00979-13
Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs
Abstract
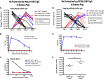
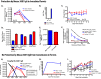
A serum hemagglutination inhibition (HAI) titer of 40 or greater is thought to be associated with reduced influenza virus pathogenesis in humans and is often used as a correlate of protection in influenza vaccine studies. We have previously demonstrated that intramuscular vaccination of guinea pigs with inactivated influenza virus generates HAI titers greater than 300 but does not protect vaccinated animals from becoming infected with influenza virus by transmission from an infected cage mate. Only guinea pigs intranasally inoculated with a live influenza virus or a live attenuated virus vaccine, prior to challenge, were protected from transmission (A. C. Lowen et al., J. Virol. 83:2803-2818, 2009.). Because the serum HAI titer is mostly determined by IgG content, these results led us to speculate that prevention of viral transmission may require IgA antibodies or cellular immune responses. To evaluate this hypothesis, guinea pigs and ferrets were administered a potent, neutralizing mouse IgG monoclonal antibody, 30D1 (Ms 30D1 IgG), against the A/California/04/2009 (H1N1) virus hemagglutinin and exposed to respiratory droplets from animals infected with this virus. Even though HAI titers were greater than 160 1 day postadministration, Ms 30D1 IgG did not prevent airborne transmission to passively immunized recipient animals. In contrast, intramuscular administration of recombinant 30D1 IgA (Ms 30D1 IgA) prevented transmission to 88% of recipient guinea pigs, and Ms 30D1 IgA was detected in animal nasal washes. Ms 30D1 IgG administered intranasally also prevented transmission, suggesting the importance of mucosal immunity in preventing influenza virus transmission. Collectively, our data indicate that IgG antibodies may prevent pathogenesis associated with influenza virus infection but do not protect from virus infection by airborne transmission, while IgA antibodies are more important for preventing transmission of influenza viruses.
Figures




References
-
- Renegar KB, Small PA, Boykins LG, Wright PF. 2004. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173:1978–1986 - PubMed
-
- Mazanec MB, Lamm ME, Lyn D, Portner A, Nedrud JG. 1992. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 23:1–12 - PubMed
-
- Johansen FE, Braathen R, Brandtzaeg P. 2000. Role of J chain in secretory immunoglobulin formation. Scand. J. Immunol. 52:240–248 - PubMed
-
- Scherle PA, Palladino G, Gerhard W. 1992. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148:212–217 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

