In vivo bioluminescent imaging of influenza a virus infection and characterization of novel cross-protective monoclonal antibodies
- PMID: 23698304
- PMCID: PMC3719835
- DOI: 10.1128/JVI.00969-13
In vivo bioluminescent imaging of influenza a virus infection and characterization of novel cross-protective monoclonal antibodies
Abstract
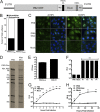
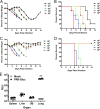
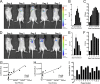
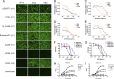
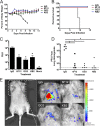
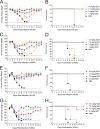
Influenza A virus is a major human pathogen responsible for seasonal epidemics as well as pandemic outbreaks. Due to the continuing burden on human health, the need for new tools to study influenza virus pathogenesis as well as to evaluate new therapeutics is paramount. We report the development of a stable, replication-competent luciferase reporter influenza A virus that can be used for in vivo imaging of viral replication. This imaging is noninvasive and allows for the longitudinal monitoring of infection in living animals. We used this tool to characterize novel monoclonal antibodies that bind the conserved stalk domain of the viral hemagglutinin of H1 and H5 subtypes and protect mice from lethal disease. The use of luciferase reporter influenza viruses allows for new mechanistic studies to expand our knowledge of virus-induced disease and provides a new quantitative method to evaluate future antiviral therapies.
Figures
References
-
- Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA
-
- Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. 2012. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr. Opin. Virol. 2:276–286 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

