β-Defensins activate human mast cells via Mas-related gene X2
- PMID: 23698749
- PMCID: PMC3691353
- DOI: 10.4049/jimmunol.1300023
β-Defensins activate human mast cells via Mas-related gene X2
Abstract
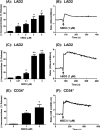
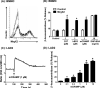
Human β-defensins (hBDs) stimulate degranulation in rat peritoneal mast cells in vitro and cause increased vascular permeability in rats in vivo. In this study, we sought to determine whether hBDs activate murine and human mast cells and to delineate the mechanisms of their regulation. hBD2 and hBD3 did not induce degranulation in murine peritoneal or bone marrow-derived mast cells (BMMC) in vitro and had no effect on vascular permeability in vivo. By contrast, these peptides induced sustained Ca(2+) mobilization and substantial degranulation in human mast cells, with hBD3 being more potent. Pertussis toxin (PTx) had no effect on hBD-induced Ca(2+) mobilization, but La(3+) and 2-aminoethoxydiphenyl borate (a dual inhibitor of inositol 1,4,5-triphosphate receptor and transient receptor potential channels) caused substantial inhibition of this response. Interestingly, degranulation induced by hBDs was substantially inhibited by PTx, La(3+), or 2-aminoethoxydiphenyl borate. Whereas human mast cells endogenously express G protein-coupled receptor, Mas-related gene X2 (MrgX2), rat basophilic leukemia, RBL-2H3 cells, and murine BMMCs do not. Silencing the expression of MrgX2 in human mast cells inhibited hBD-induced degranulation, but had no effect on anaphylatoxin C3a-induced response. Furthermore, ectopic expression of MrgX2 in RBL-2H3 and murine BMMCs rendered these cells responsive to hBDs for degranulation. This study demonstrates that hBDs activate human mast cells via MrgX2, which couples to both PTx-sensitive and insensitive signaling pathways most likely involving Gαq and Gαi to induce degranulation. Furthermore, murine mast cells are resistant to hBDs for degranulation, and this reflects the absence of MrgX2 in these cells.
Figures

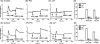



Similar articles
-
G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2.Eur J Pharmacol. 2011 Oct 1;668(1-2):299-304. doi: 10.1016/j.ejphar.2011.06.027. Epub 2011 Jul 3. Eur J Pharmacol. 2011. PMID: 21741965 Free PMC article.
-
Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization.J Biol Chem. 2011 Dec 30;286(52):44739-49. doi: 10.1074/jbc.M111.277152. Epub 2011 Nov 8. J Biol Chem. 2011. PMID: 22069323 Free PMC article.
-
Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties.Oncotarget. 2015 Oct 6;6(30):28573-87. doi: 10.18632/oncotarget.5611. Oncotarget. 2015. PMID: 26378047 Free PMC article.
-
The Origin, Expression, Function and Future Research Focus of a G Protein-coupled Receptor, Mas-related Gene X2 (MrgX2).Prog Histochem Cytochem. 2015 Jul;50(1-2):11-7. doi: 10.1016/j.proghi.2015.06.001. Epub 2015 Jun 8. Prog Histochem Cytochem. 2015. PMID: 26106044 Review.
-
Human β-defensins and their synthetic analogs: Natural defenders and prospective new drugs of oral health.Life Sci. 2024 Jun 1;346:122591. doi: 10.1016/j.lfs.2024.122591. Epub 2024 Mar 26. Life Sci. 2024. PMID: 38548013 Review.
Cited by
-
MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions.Pharmacol Res Perspect. 2019 Dec 2;7(6):e00547. doi: 10.1002/prp2.547. eCollection 2019 Dec. Pharmacol Res Perspect. 2019. PMID: 31832205 Free PMC article.
-
Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2).Cells. 2021 Apr 27;10(5):1033. doi: 10.3390/cells10051033. Cells. 2021. PMID: 33925682 Free PMC article. Review.
-
Neuroimmune communication in allergic rhinitis.Front Neurol. 2023 Dec 21;14:1282130. doi: 10.3389/fneur.2023.1282130. eCollection 2023. Front Neurol. 2023. PMID: 38178883 Free PMC article. Review.
-
Immunomodulatory and Allergenic Properties of Antimicrobial Peptides.Int J Mol Sci. 2022 Feb 24;23(5):2499. doi: 10.3390/ijms23052499. Int J Mol Sci. 2022. PMID: 35269641 Free PMC article. Review.
-
Human β-Defensin 2 Expression in Oral Epithelium: Potential Therapeutic Targets in Oral Lichen Planus.Int J Mol Sci. 2019 Apr 10;20(7):1780. doi: 10.3390/ijms20071780. Int J Mol Sci. 2019. PMID: 30974892 Free PMC article.
References
-
- Tohidnezhad M, Varoga D, Podschun R, Wruck CJ, Seekamp A, Brandenburg LO, Pufe T, Lippross S. Thrombocytes are effectors of the innate immune system releasing human beta defensin-3. Injury. 2011;42:682–686. - PubMed
-
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

