Role of pore-forming toxins in bacterial infectious diseases
- PMID: 23699254
- PMCID: PMC3668673
- DOI: 10.1128/MMBR.00052-12
Role of pore-forming toxins in bacterial infectious diseases
Abstract
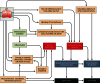
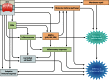
Pore-forming toxins (PFTs) are the most common bacterial cytotoxic proteins and are required for virulence in a large number of important pathogens, including Streptococcus pneumoniae, group A and B streptococci, Staphylococcus aureus, Escherichia coli, and Mycobacterium tuberculosis. PFTs generally disrupt host cell membranes, but they can have additional effects independent of pore formation. Substantial effort has been devoted to understanding the molecular mechanisms underlying the functions of certain model PFTs. Likewise, specific host pathways mediating survival and immune responses in the face of toxin-mediated cellular damage have been delineated. However, less is known about the overall functions of PFTs during infection in vivo. This review focuses on common themes in the area of PFT biology, with an emphasis on studies addressing the roles of PFTs in in vivo and ex vivo models of colonization or infection. Common functions of PFTs include disruption of epithelial barrier function and evasion of host immune responses, which contribute to bacterial growth and spreading. The widespread nature of PFTs make this group of toxins an attractive target for the development of new virulence-targeted therapies that may have broad activity against human pathogens.
Figures






References
-
- Woodford N, Livermore DM. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 59(Suppl 1):S4–S16 - PubMed
-
- Huttner B, Goossens H, Verheij T, Harbarth S. 2010. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect. Dis. 10:17–31 - PubMed
-
- Alouf JE. 2003. Molecular features of the cytolytic pore-forming bacterial protein toxins. Folia Microbiol. (Prague) 48:5–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

