Toxin plasmids of Clostridium perfringens
- PMID: 23699255
- PMCID: PMC3668675
- DOI: 10.1128/MMBR.00062-12
Toxin plasmids of Clostridium perfringens
Abstract
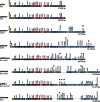
In both humans and animals, Clostridium perfringens is an important cause of histotoxic infections and diseases originating in the intestines, such as enteritis and enterotoxemia. The virulence of this Gram-positive, anaerobic bacterium is heavily dependent upon its prolific toxin-producing ability. Many of the ∼16 toxins produced by C. perfringens are encoded by large plasmids that range in size from ∼45 kb to ∼140 kb. These plasmid-encoded toxins are often closely associated with mobile elements. A C. perfringens strain can carry up to three different toxin plasmids, with a single plasmid carrying up to three distinct toxin genes. Molecular Koch's postulate analyses have established the importance of several plasmid-encoded toxins when C. perfringens disease strains cause enteritis or enterotoxemias. Many toxin plasmids are closely related, suggesting a common evolutionary origin. In particular, most toxin plasmids and some antibiotic resistance plasmids of C. perfringens share an ∼35-kb region containing a Tn916-related conjugation locus named tcp (transfer of clostridial plasmids). This tcp locus can mediate highly efficient conjugative transfer of these toxin or resistance plasmids. For example, conjugative transfer of a toxin plasmid from an infecting strain to C. perfringens normal intestinal flora strains may help to amplify and prolong an infection. Therefore, the presence of toxin genes on conjugative plasmids, particularly in association with insertion sequences that may mobilize these toxin genes, likely provides C. perfringens with considerable virulence plasticity and adaptability when it causes diseases originating in the gastrointestinal tract.
Figures












References
-
- McClane BA, Robertson SL, Li J. 2013. Clostridium perfringens, p 465–489 In Doyle MP, Buchanan RL. (ed), Food microbiology: fundamentals and frontiers, 4th ed ASM Press, Washington, DC
-
- Labbe RG. 1989. Clostridium perfringens, p 192–234 In Doyle MP. (ed), Foodborne bacterial pathogens. Marcel Decker, New York, NY
-
- McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. 2006. The enterotoxic clostridia, p 688–752 In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed Springer, New York, NY
-
- Rood JI. 2006. Clostridium perfringens and histotoxic disease, p 753–770 In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed Springer, New York, NY
-
- Titball RW, Rood JI. 2002. Clostridium perfringens: wound infections, p 1875–1903 In Sussman M. (ed), Molecular medical microbiology. Academic Press, London, England
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

