Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease
- PMID: 23699256
- PMCID: PMC3668674
- DOI: 10.1128/MMBR.00056-12
Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease
Abstract
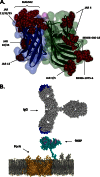
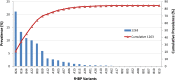
Neisseria meningitidis is a Gram-negative microorganism that exists exclusively in humans and can cause devastating invasive disease. Although capsular polysaccharide-based vaccines against serogroups A, C, Y, and W135 are widely available, the pathway to a broadly protective vaccine against serogroup B has been more complex. The last 11 years has seen the discovery and development of the N. meningitidis serogroup B (MnB) outer membrane protein factor H binding protein (fHBP) as a vaccine component. Since the initial discovery of fHBP, a tremendous amount of work has accumulated on the diversity, structure, and regulation of this important protein. fHBP has proved to be a virulence factor for N. meningitidis and a target for functional bactericidal antibodies. fHBP is critical for survival of meningococci in the human host, as it is responsible for the primary interaction with human factor H (fH). Binding of hfH by the meningococcus serves to downregulate the host alternative complement pathway and helps the organism evade host innate immunity. Preclinical studies have shown that an fHBP-based vaccine can elicit serum bactericidal antibodies capable of killing MnB, and the vaccine has shown very encouraging results in human clinical trials. This report reviews our current knowledge of fHBP. In particular, we discuss the recent advances in our understanding of fHBP, its importance to N. meningitidis, and its potential role as a vaccine for preventing MnB disease.
Figures




References
-
- Stephens DS. 2007. Conquering the meningococcus. FEMS Microbiol. Rev. 31:3–14 - PubMed
-
- Wilder-Smith A. 2009. Meningococcal vaccines: a neglected topic in travel medicine? Expert Rev. Vaccines 8:1343–1350 - PubMed
-
- Claus H, Maiden MC, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, Hessler F, Frosch M, Vogel U. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263–1271 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

