Pyrophosphate-fueled Na+ and H+ transport in prokaryotes
- PMID: 23699258
- PMCID: PMC3668671
- DOI: 10.1128/MMBR.00003-13
Pyrophosphate-fueled Na+ and H+ transport in prokaryotes
Erratum in
- Microbiol Mol Biol Rev. 2013 Sep;77(3):540
Abstract
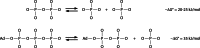
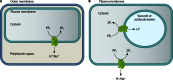
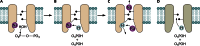
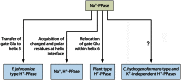
In its early history, life appeared to depend on pyrophosphate rather than ATP as the source of energy. Ancient membrane pyrophosphatases that couple pyrophosphate hydrolysis to active H(+) transport across biological membranes (H(+)-pyrophosphatases) have long been known in prokaryotes, plants, and protists. Recent studies have identified two evolutionarily related and widespread prokaryotic relics that can pump Na(+) (Na(+)-pyrophosphatase) or both Na(+) and H(+) (Na(+),H(+)-pyrophosphatase). Both these transporters require Na(+) for pyrophosphate hydrolysis and are further activated by K(+). The determination of the three-dimensional structures of H(+)- and Na(+)-pyrophosphatases has been another recent breakthrough in the studies of these cation pumps. Structural and functional studies have highlighted the major determinants of the cation specificities of membrane pyrophosphatases and their potential use in constructing transgenic stress-resistant organisms.
Figures
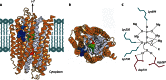
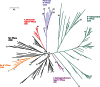
References
-
- Baltscheffsky H. 1996. Energy conversion leading to the origin and early evolution of life: did inorganic pyrophosphate precede adenosine triphosphate?, p 1–9 In Baltscheffsky H. (ed), Origin and evolution of biological energy conversion. VCH, New York, NY
-
- Heinonen JK. 2001. Biological role of inorganic pyrophosphate. Kluwer Academic Publishers, London, United Kingdom
-
- Baltscheffsky H, Von Stedingk LV, Heldt HW, Klingenberg M. 1966. Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science 153:1120–1122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

