Acyltransferases in bacteria
- PMID: 23699259
- PMCID: PMC3668668
- DOI: 10.1128/MMBR.00010-13
Acyltransferases in bacteria
Abstract
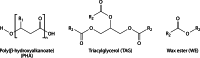
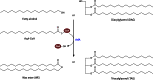
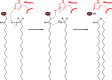
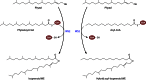
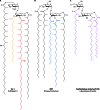
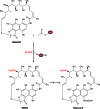
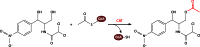
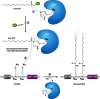
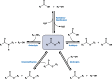
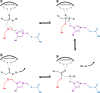
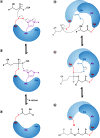
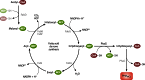
Long-chain-length hydrophobic acyl residues play a vital role in a multitude of essential biological structures and processes. They build the inner hydrophobic layers of biological membranes, are converted to intracellular storage compounds, and are used to modify protein properties or function as membrane anchors, to name only a few functions. Acyl thioesters are transferred by acyltransferases or transacylases to a variety of different substrates or are polymerized to lipophilic storage compounds. Lipases represent another important enzyme class dealing with fatty acyl chains; however, they cannot be regarded as acyltransferases in the strict sense. This review provides a detailed survey of the wide spectrum of bacterial acyltransferases and compares different enzyme families in regard to their catalytic mechanisms. On the basis of their studied or assumed mechanisms, most of the acyl-transferring enzymes can be divided into two groups. The majority of enzymes discussed in this review employ a conserved acyltransferase motif with an invariant histidine residue, followed by an acidic amino acid residue, and their catalytic mechanism is characterized by a noncovalent transition state. In contrast to that, lipases rely on completely different mechanism which employs a catalytic triad and functions via the formation of covalent intermediates. This is, for example, similar to the mechanism which has been suggested for polyester synthases. Consequently, although the presented enzyme types neither share homology nor have a common three-dimensional structure, and although they deal with greatly varying molecule structures, this variety is not reflected in their mechanisms, all of which rely on a catalytically active histidine residue.
Figures






Similar articles
-
An essential histidine residue required for fatty acylation and acyl transfer by myristoyltransferase from luminescent bacteria.J Biol Chem. 1994 Mar 4;269(9):6683-8. J Biol Chem. 1994. PMID: 8120025
-
Crystal structure of a membrane-bound O-acyltransferase.Nature. 2018 Oct;562(7726):286-290. doi: 10.1038/s41586-018-0568-2. Epub 2018 Oct 3. Nature. 2018. PMID: 30283133 Free PMC article.
-
Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: identification of catalytic residues.Biochem J. 2003 Sep 1;374(Pt 2):413-21. doi: 10.1042/BJ20030431. Biochem J. 2003. PMID: 12924980 Free PMC article.
-
Polyester synthases: natural catalysts for plastics.Biochem J. 2003 Nov 15;376(Pt 1):15-33. doi: 10.1042/BJ20031254. Biochem J. 2003. PMID: 12954080 Free PMC article. Review.
-
Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells.J Biochem. 1997 Jul;122(1):1-16. doi: 10.1093/oxfordjournals.jbchem.a021715. J Biochem. 1997. PMID: 9276665 Review.
Cited by
-
Elucidating the Antimycobacterial Mechanism of Action of Ciprofloxacin Using Metabolomics.Microorganisms. 2021 May 28;9(6):1158. doi: 10.3390/microorganisms9061158. Microorganisms. 2021. PMID: 34071153 Free PMC article.
-
Use of limited proteolysis and mutagenesis to identify folding domains and sequence motifs critical for wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase activity.Appl Environ Microbiol. 2014 Feb;80(3):1132-41. doi: 10.1128/AEM.03433-13. Epub 2013 Dec 2. Appl Environ Microbiol. 2014. PMID: 24296496 Free PMC article.
-
Acylated plastoquinone is a novel neutral lipid accumulated in cyanobacteria.PNAS Nexus. 2023 Mar 22;2(5):pgad092. doi: 10.1093/pnasnexus/pgad092. eCollection 2023 May. PNAS Nexus. 2023. PMID: 37152674 Free PMC article.
-
The WSD-type wax ester synthase is widely conserved in streptophytes and crucial for floral organ formation under high humidity in land plants.J Plant Res. 2025 May;138(3):497-509. doi: 10.1007/s10265-025-01628-6. Epub 2025 Apr 1. J Plant Res. 2025. PMID: 40169465 Free PMC article.
-
Functional Characterization of Physcomitrella patens Glycerol-3-Phosphate Acyltransferase 9 and an Increase in Seed Oil Content in Arabidopsis by Its Ectopic Expression.Plants (Basel). 2019 Aug 13;8(8):284. doi: 10.3390/plants8080284. Plants (Basel). 2019. PMID: 31412690 Free PMC article.
References
-
- White SW, Zheng J, Zhang YM, Rock CO. 2005. The structural biology of the type II fatty acid biosynthesis. Annu. Rev. Biochem. 74:791–831 - PubMed
-
- Chan DI, Vogel HJ. 2010. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 430:1–19 - PubMed
-
- Murphy DJ. 1993. Structure, function and biogenesis of storage lipid bodies and oleosins in plants. Prog. Lipid Res. 32:247–280 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

