Acyltransferases in bacteria
- PMID: 23699259
- PMCID: PMC3668668
- DOI: 10.1128/MMBR.00010-13
Acyltransferases in bacteria
Abstract
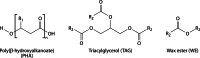
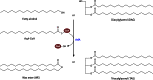
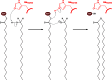
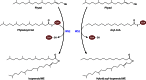
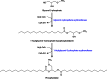
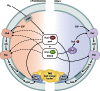
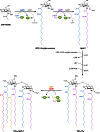
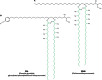
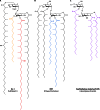
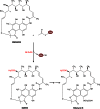
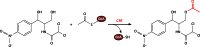
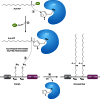
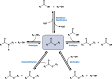
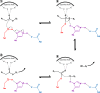
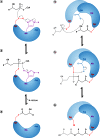
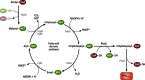
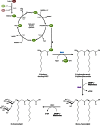
Long-chain-length hydrophobic acyl residues play a vital role in a multitude of essential biological structures and processes. They build the inner hydrophobic layers of biological membranes, are converted to intracellular storage compounds, and are used to modify protein properties or function as membrane anchors, to name only a few functions. Acyl thioesters are transferred by acyltransferases or transacylases to a variety of different substrates or are polymerized to lipophilic storage compounds. Lipases represent another important enzyme class dealing with fatty acyl chains; however, they cannot be regarded as acyltransferases in the strict sense. This review provides a detailed survey of the wide spectrum of bacterial acyltransferases and compares different enzyme families in regard to their catalytic mechanisms. On the basis of their studied or assumed mechanisms, most of the acyl-transferring enzymes can be divided into two groups. The majority of enzymes discussed in this review employ a conserved acyltransferase motif with an invariant histidine residue, followed by an acidic amino acid residue, and their catalytic mechanism is characterized by a noncovalent transition state. In contrast to that, lipases rely on completely different mechanism which employs a catalytic triad and functions via the formation of covalent intermediates. This is, for example, similar to the mechanism which has been suggested for polyester synthases. Consequently, although the presented enzyme types neither share homology nor have a common three-dimensional structure, and although they deal with greatly varying molecule structures, this variety is not reflected in their mechanisms, all of which rely on a catalytically active histidine residue.
Figures






References
-
- White SW, Zheng J, Zhang YM, Rock CO. 2005. The structural biology of the type II fatty acid biosynthesis. Annu. Rev. Biochem. 74:791–831 - PubMed
-
- Chan DI, Vogel HJ. 2010. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 430:1–19 - PubMed
-
- Murphy DJ. 1993. Structure, function and biogenesis of storage lipid bodies and oleosins in plants. Prog. Lipid Res. 32:247–280 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

