SHIP2 regulates epithelial cell polarity through its lipid product, which binds to Dlg1, a pathway subverted by hepatitis C virus core protein
- PMID: 23699395
- PMCID: PMC3708724
- DOI: 10.1091/mbc.E12-08-0626
SHIP2 regulates epithelial cell polarity through its lipid product, which binds to Dlg1, a pathway subverted by hepatitis C virus core protein
Abstract
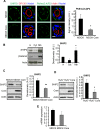
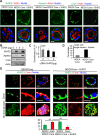
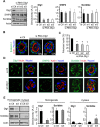
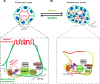
The main targets of hepatitis C virus (HCV) are hepatocytes, the highly polarized cells of the liver, and all the steps of its life cycle are tightly dependent on host lipid metabolism. The interplay between polarity and lipid metabolism in HCV infection has been poorly investigated. Signaling lipids, such as phosphoinositides (PIs), play a vital role in polarity, which depends on the distribution and expression of PI kinases and PI phosphatases. In this study, we report that HCV core protein, expressed in Huh7 and Madin-Darby canine kidney (MDCK) cells, disrupts apicobasal polarity. This is associated with decreased expression of the polarity protein Dlg1 and the PI phosphatase SHIP2, which converts phosphatidylinositol 3,4,5-trisphosphate into phosphatidylinositol 4,5-bisphosphate (PtdIns(3,4)P2). SHIP2 is mainly localized at the basolateral membrane of polarized MDCK cells. In addition, PtdIns(3,4)P2 is able to bind to Dlg1. SHIP2 small interfering RNA or its catalytically dead mutant disrupts apicobasal polarity, similar to HCV core. In core-expressing cells, RhoA activity is inhibited, whereas Rac1 is activated. Of interest, SHIP2 expression rescues polarity, RhoA activation, and restricted core level in MDCK cells. We conclude that SHIP2 is an important regulator of polarity, which is subverted by HCV in epithelial cells. It is suggested that SHIP2 could be a promising target for anti-HCV treatment.
Figures





Similar articles
-
PI3K/SHIP2/PTEN pathway in cell polarity and hepatitis C virus pathogenesis.World J Hepatol. 2017 Jan 8;9(1):18-29. doi: 10.4254/wjh.v9.i1.18. World J Hepatol. 2017. PMID: 28105255 Free PMC article. Review.
-
SHIP2 Regulates Lumen Generation, Cell Division, and Ciliogenesis through the Control of Basolateral to Apical Lumen Localization of Aurora A and HEF 1.Cell Rep. 2016 Dec 6;17(10):2738-2752. doi: 10.1016/j.celrep.2016.11.033. Cell Rep. 2016. PMID: 27926875
-
Dual role of SRC homology domain 2-containing inositol phosphatase 2 in the regulation of platelet-derived growth factor and insulin-like growth factor I signaling in rat vascular smooth muscle cells.Endocrinology. 2003 Sep;144(9):4204-14. doi: 10.1210/en.2003-0190. Endocrinology. 2003. PMID: 12933696
-
Lipid phosphatases SKIP and SHIP2 regulate fibronectin-dependent cell migration in glioblastoma.FEBS J. 2019 Mar;286(6):1120-1135. doi: 10.1111/febs.14769. Epub 2019 Feb 16. FEBS J. 2019. PMID: 30695232
-
Phosphoinositide 5-phosphatase activities control cell motility in glioblastoma: Two phosphoinositides PI(4,5)P2 and PI(3,4)P2 are involved.Adv Biol Regul. 2018 Jan;67:40-48. doi: 10.1016/j.jbior.2017.09.001. Epub 2017 Sep 5. Adv Biol Regul. 2018. PMID: 28916189 Review.
Cited by
-
Membrane Lipids in Epithelial Polarity: Sorting out the PIPs.Front Cell Dev Biol. 2022 May 31;10:893960. doi: 10.3389/fcell.2022.893960. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35712665 Free PMC article. Review.
-
JAM-A regulates cortical dynein localization through Cdc42 to control planar spindle orientation during mitosis.Nat Commun. 2015 Aug 26;6:8128. doi: 10.1038/ncomms9128. Nat Commun. 2015. PMID: 26306570 Free PMC article.
-
PDZ Domains as Drug Targets.Adv Ther (Weinh). 2019 Jul;2(7):1800143. doi: 10.1002/adtp.201800143. Epub 2019 Apr 24. Adv Ther (Weinh). 2019. PMID: 32313833 Free PMC article. Review.
-
PI3K/SHIP2/PTEN pathway in cell polarity and hepatitis C virus pathogenesis.World J Hepatol. 2017 Jan 8;9(1):18-29. doi: 10.4254/wjh.v9.i1.18. World J Hepatol. 2017. PMID: 28105255 Free PMC article. Review.
-
Structural and functional hepatocyte polarity and liver disease.J Hepatol. 2015 Oct;63(4):1023-37. doi: 10.1016/j.jhep.2015.06.015. Epub 2015 Jun 24. J Hepatol. 2015. PMID: 26116792 Free PMC article. Review.
References
-
- Alvisi G, Madan V, Bartenschlager R. Hepatitis C virus and host cell lipids: an intimate connection. RNA Biol. 2011;8:258–269. - PubMed
-
- Backers K, Blero D, Paternotte N, Zhang J, Erneux C. The termination of PI3K signalling by SHIP1 and SHIP2 inositol 5-phosphatases. Adv Enzyme Regul. 2003;43:15–28. - PubMed
-
- Bartenschlager R, Penin F, Lohmann V, André P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

