Balance of calcineurin Aα and CDK5 activities sets release probability at nerve terminals
- PMID: 23699505
- PMCID: PMC3808255
- DOI: 10.1523/JNEUROSCI.4288-12.2013
Balance of calcineurin Aα and CDK5 activities sets release probability at nerve terminals
Abstract
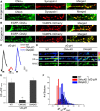
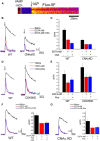
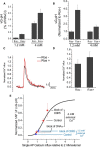
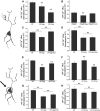
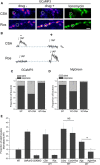
The control of neurotransmitter release at nerve terminals is of profound importance for neurological function and provides a powerful control system in neural networks. We show that the balance of enzymatic activities of the α isoform of the phosphatase calcineurin (CNAα) and the kinase cyclin-dependent kinase 5 (CDK5) has a dramatic influence over single action potential (AP)-driven exocytosis at nerve terminals. Acute or chronic loss of these enzymatic activities results in a sevenfold impact on single AP-driven exocytosis. We demonstrate that this control is mediated almost entirely through Cav2.2 (N-type) voltage-gated calcium channels as blocking these channels with a peptide toxin eliminates modulation by these enzymes. We found that a fraction of nerve terminals are kept in a presynaptically silent state with no measurable Ca(2+) influx driven by single AP stimuli attributable to the balance of CNAα and CDK5 activities because blockade of either CNAα or CDK5 activity changes the proportion of presynaptically silent nerve terminals. Thus, CNAα and CDK5 enzymatic activities are key determinants of release probability.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
