Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation
- PMID: 23699511
- PMCID: PMC3712504
- DOI: 10.1523/JNEUROSCI.0914-13.2013
Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation
Abstract
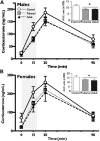
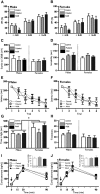
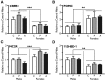
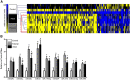
Neuropsychiatric disease frequently presents with an underlying hyporeactivity or hyperreactivity of the HPA stress axis, suggesting an exceptional vulnerability of this circuitry to external perturbations. Parental lifetime exposures to environmental challenges are associated with increased offspring neuropsychiatric disease risk, and likely contribute to stress dysregulation. While maternal influences have been extensively examined, much less is known regarding the specific role of paternal factors. To investigate the potential mechanisms by which paternal stress may contribute to offspring hypothalamic-pituitary-adrenal (HPA) axis dysregulation, we exposed mice to 6 weeks of chronic stress before breeding. As epidemiological studies support variation in paternal germ cell susceptibility to reprogramming across the lifespan, male stress exposure occurred either throughout puberty or in adulthood. Remarkably, offspring of sires from both paternal stress groups displayed significantly reduced HPA stress axis responsivity. Gene set enrichment analyses in offspring stress regulating brain regions, the paraventricular nucleus (PVN) and the bed nucleus of stria terminalis, revealed global pattern changes in transcription suggestive of epigenetic reprogramming and consistent with altered offspring stress responsivity, including increased expression of glucocorticoid-responsive genes in the PVN. In examining potential epigenetic mechanisms of germ cell transmission, we found robust changes in sperm microRNA (miR) content, where nine specific miRs were significantly increased in both paternal stress groups. Overall, these results demonstrate that paternal experience across the lifespan can induce germ cell epigenetic reprogramming and impact offspring HPA stress axis regulation, and may therefore offer novel insight into factors influencing neuropsychiatric disease risk.
Figures
Comment in
-
Epigenetics: keeping it in the family.Nat Rev Neurosci. 2013 Jul;14(7):458-9. doi: 10.1038/nrn3536. Epub 2013 Jun 12. Nat Rev Neurosci. 2013. PMID: 23756636 No abstract available.
Similar articles
-
Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress.Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13699-704. doi: 10.1073/pnas.1508347112. Epub 2015 Oct 19. Proc Natl Acad Sci U S A. 2015. PMID: 26483456 Free PMC article.
-
Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring.Transl Psychiatry. 2016 Jun 14;6(6):e837. doi: 10.1038/tp.2016.109. Transl Psychiatry. 2016. PMID: 27300263 Free PMC article.
-
Paternal environmental enrichment transgenerationally alters affective behavioral and neuroendocrine phenotypes.Psychoneuroendocrinology. 2017 Mar;77:225-235. doi: 10.1016/j.psyneuen.2016.11.013. Epub 2017 Jan 4. Psychoneuroendocrinology. 2017. PMID: 28104556
-
Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity.Stress. 2017 Sep;20(5):476-494. doi: 10.1080/10253890.2017.1369523. Epub 2017 Aug 31. Stress. 2017. PMID: 28859530 Free PMC article. Review.
-
Germ Cell Origins of Posttraumatic Stress Disorder Risk: The Transgenerational Impact of Parental Stress Experience.Biol Psychiatry. 2015 Sep 1;78(5):307-14. doi: 10.1016/j.biopsych.2015.03.018. Epub 2015 Mar 23. Biol Psychiatry. 2015. PMID: 25895429 Free PMC article. Review.
Cited by
-
Sperm histone H3 lysine 4 trimethylation is altered in a genetic mouse model of transgenerational epigenetic inheritance.Nucleic Acids Res. 2020 Nov 18;48(20):11380-11393. doi: 10.1093/nar/gkaa712. Nucleic Acids Res. 2020. PMID: 33068438 Free PMC article.
-
Sperm microRNA Content Is Altered in a Mouse Model of Male Obesity, but the Same Suite of microRNAs Are Not Altered in Offspring's Sperm.PLoS One. 2016 Nov 4;11(11):e0166076. doi: 10.1371/journal.pone.0166076. eCollection 2016. PLoS One. 2016. PMID: 27814400 Free PMC article.
-
Work-Related Stress, Physio-Pathological Mechanisms, and the Influence of Environmental Genetic Factors.Int J Environ Res Public Health. 2019 Oct 21;16(20):4031. doi: 10.3390/ijerph16204031. Int J Environ Res Public Health. 2019. PMID: 31640269 Free PMC article.
-
Single-cell multi-omics of human preimplantation embryos shows susceptibility to glucocorticoids.Genome Res. 2022 Sep 27;32(9):1627-1641. doi: 10.1101/gr.276665.122. Genome Res. 2022. PMID: 35948369 Free PMC article.
-
Peripubertal Stress With Social Support Promotes Resilience in the Face of Aging.Endocrinology. 2016 May;157(5):2002-14. doi: 10.1210/en.2015-1876. Epub 2016 Mar 4. Endocrinology. 2016. PMID: 26943365 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
