Neuronal mechanisms of respiratory pattern generation are evolutionary conserved
- PMID: 23699521
- PMCID: PMC6705030
- DOI: 10.1523/JNEUROSCI.0299-13.2013
Neuronal mechanisms of respiratory pattern generation are evolutionary conserved
Abstract
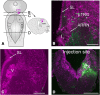
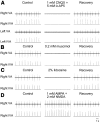
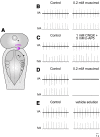
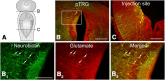
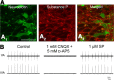
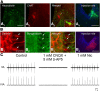
A brainstem region, the paratrigeminal respiratory group (pTRG), has been suggested to play a crucial role in the respiratory rhythm generation in lampreys. However, a detailed characterization of the pTRG region is lacking. The present study performed on isolated brainstem preparations of adult lampreys provides a more precise localization of the pTRG region with regard to both connectivity and neurochemical markers. pTRG neurons projecting to the vagal motoneuronal pool were identified in a restricted area of the rostral rhombencephalon at the level of the isthmic Müller cell I1 close to sulcus limitans of His. Unilateral microinjections of lidocaine, muscimol, or glutamate antagonists into the pTRG inhibited completely the bilateral respiratory activity. In contrast, microinjections of glutamate agonists enhanced the respiratory activity, suggesting that this region is critical for the respiratory pattern generation. The retrogradely labeled pTRG neurons are glutamatergic and surrounded by terminals with intense substance P immunoreactivity. Cholinergic neurons were seen close to, and intermingled with, pTRG neurons. In addition, α-bungarotoxin binding sites (indicating nicotinic receptors) were found throughout the pTRG area and particularly on the soma of these neurons. During apnea, induced by blockade of ionotropic glutamate receptors within the same region, microinjections of 1 μm substance P or 1 mm nicotine into the pTRG restored rhythmic respiratory activity. The results emphasize the close similarities between the pTRG and the mammalian pre-Bötzinger complex as a crucial site for respiratory rhythmogenesis. We conclude that some basic features of the excitatory neurons proposed to generate respiratory rhythms are conserved throughout evolution.
Figures
Similar articles
-
GABAergic and glycinergic inputs modulate rhythmogenic mechanisms in the lamprey respiratory network.J Physiol. 2014 Apr 15;592(8):1823-38. doi: 10.1113/jphysiol.2013.268086. Epub 2014 Feb 3. J Physiol. 2014. PMID: 24492840 Free PMC article.
-
Identification of a cholinergic modulatory and rhythmogenic mechanism within the lamprey respiratory network.J Neurosci. 2011 Sep 14;31(37):13323-32. doi: 10.1523/JNEUROSCI.2764-11.2011. J Neurosci. 2011. PMID: 21917815 Free PMC article.
-
Inhibitory control of ascending glutamatergic projections to the lamprey respiratory rhythm generator.Neuroscience. 2016 Jun 21;326:126-140. doi: 10.1016/j.neuroscience.2016.03.063. Epub 2016 Apr 4. Neuroscience. 2016. PMID: 27058146
-
Neural mechanisms underlying respiratory rhythm generation in the lamprey.Respir Physiol Neurobiol. 2016 Apr;224:17-26. doi: 10.1016/j.resp.2014.09.003. Epub 2014 Sep 16. Respir Physiol Neurobiol. 2016. PMID: 25220696 Review.
-
Initiation of locomotion in lampreys.Brain Res Rev. 2008 Jan;57(1):172-82. doi: 10.1016/j.brainresrev.2007.07.016. Epub 2007 Aug 22. Brain Res Rev. 2008. PMID: 17916380 Review.
Cited by
-
Genomic discovery of ion channel genes in the central nervous system of the lamprey Petromyzon marinus.Mar Genomics. 2019 Aug;46:29-40. doi: 10.1016/j.margen.2019.03.003. Epub 2019 Mar 14. Mar Genomics. 2019. PMID: 30878501 Free PMC article.
-
GABAergic and glycinergic inputs modulate rhythmogenic mechanisms in the lamprey respiratory network.J Physiol. 2014 Apr 15;592(8):1823-38. doi: 10.1113/jphysiol.2013.268086. Epub 2014 Feb 3. J Physiol. 2014. PMID: 24492840 Free PMC article.
-
ATP and astrocytes play a prominent role in the control of the respiratory pattern generator in the lamprey.J Physiol. 2017 Dec 1;595(23):7063-7079. doi: 10.1113/JP274749. Epub 2017 Aug 8. J Physiol. 2017. PMID: 28734063 Free PMC article.
-
Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish.Elife. 2021 Mar 15;10:e63407. doi: 10.7554/eLife.63407. Elife. 2021. PMID: 33720013 Free PMC article.
-
Molecular characterization of frog vocal neurons using constellation pharmacology.J Neurophysiol. 2020 Jun 1;123(6):2297-2310. doi: 10.1152/jn.00105.2020. Epub 2020 May 6. J Neurophysiol. 2020. PMID: 32374212 Free PMC article.
References
-
- Ben-Mabrouk F, Amos LB, Tryba AK. Metabotropic glutamate receptors (mGluR5) activate transient receptor potential canonical channels to improve the regularity of the respiratory rhythm generated by the pre-Botzinger complex in mice. Eur J Neurosci. 2012;35:1725–1737. doi: 10.1111/j.1460-9568.2012.08091.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
