Protection from noise-induced hearing loss by Kv2.2 potassium currents in the central medial olivocochlear system
- PMID: 23699522
- PMCID: PMC5503134
- DOI: 10.1523/JNEUROSCI.5043-12.2013
Protection from noise-induced hearing loss by Kv2.2 potassium currents in the central medial olivocochlear system
Abstract
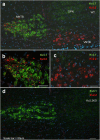
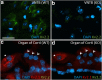
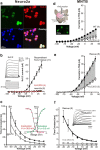
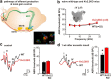
The central auditory brainstem provides an efferent projection known as the medial olivocochlear (MOC) system, which regulates the cochlear amplifier and mediates protection on exposure to loud sound. It arises from neurons of the ventral nucleus of the trapezoid body (VNTB), so control of neuronal excitability in this pathway has profound effects on hearing. The VNTB and the medial nucleus of the trapezoid body are the only sites of expression for the Kv2.2 voltage-gated potassium channel in the auditory brainstem, consistent with a specialized function of these channels. In the absence of unambiguous antagonists, we used recombinant and transgenic methods to examine how Kv2.2 contributes to MOC efferent function. Viral gene transfer of dominant-negative Kv2.2 in wild-type mice suppressed outward K(+) currents, increasing action potential (AP) half-width and reducing repetitive firing. Similarly, VNTB neurons from Kv2.2 knock-out mice (Kv2.2KO) also showed increased AP duration. Control experiments established that Kv2.2 was not expressed in the cochlea, so any changes in auditory function in the Kv2.2KO mouse must be of central origin. Further, in vivo recordings of auditory brainstem responses revealed that these Kv2.2KO mice were more susceptible to noise-induced hearing loss. We conclude that Kv2.2 regulates neuronal excitability in these brainstem nuclei by maintaining short APs and enhancing high-frequency firing. This safeguards efferent MOC firing during high-intensity sounds and is crucial in the mediation of protection after auditory overexposure.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
