The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses
- PMID: 23699527
- PMCID: PMC3692273
- DOI: 10.1523/JNEUROSCI.0301-13.2013
The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses
Abstract
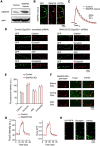
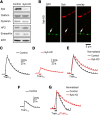
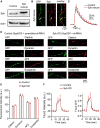
SNAP25, an essential component of the soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor (SNARE) complex that mediates exocytosis, is not considered to play a role in endocytosis, which couples to exocytosis by retrieving a similar amount of exocytosed vesicles. By knocking down SNAP25 and imaging slow endocytosis at a conventional synapse, the rat cultured hippocampal synapse, we found that SNAP25 is involved in slow, clathrin-dependent endocytosis. With similar techniques, we found that not only SNAP25, but also synaptobrevin is involved in slow endocytosis. These results provide the first evidence showing the dual role of SNAP25 and synaptobrevin in both exocytosis and slow endocytosis at conventional synapses. Such a dual role may contribute to mediate the coupling between exocytosis and clathrin-dependent endocytosis at conventional synapses, a mechanism critical for the maintenance of synaptic transmission and the normal structure of nerve terminals.
Figures

References
-
- Galas MC, Chasserot-Golaz S, Dirrig-Grosch S, Bader MF. Presence of dynamin–syntaxin complexes associated with secretory granules in adrenal chromaffin cells. J Neurochem. 2000;75:1511–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
