A study of tropomyosin's role in cardiac function and disease using thin-filament reconstituted myocardium
- PMID: 23700264
- PMCID: PMC3849125
- DOI: 10.1007/s10974-013-9343-z
A study of tropomyosin's role in cardiac function and disease using thin-filament reconstituted myocardium
Abstract
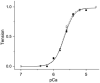
Tropomyosin (Tm) is the key regulatory component of the thin-filament and plays a central role in the cardiac muscle's cooperative activation mechanism. Many mutations of cardiac Tm are related to hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), and left ventricular noncompaction (LVNC). Using the thin-filament extraction/reconstitution technique, we are able to incorporate various Tm mutants and protein isoforms into a muscle fiber environment to study their roles in Ca(2+) regulation, cross-bridge kinetics, and force generation. The thin-filament reconstitution technique poses several advantages compared to other in vitro and in vivo methods: (1) Tm mutants and isoforms are placed into the real muscle fiber environment to exhibit their effect on a level much higher than simple protein complexes; (2) only the primary and immediate effects of Tm mutants are studied in the thin-filament reconstituted myocardium; (3) lethal mutants of Tm can be studied without causing a problem; and (4) inexpensive. In transgenic models, various secondary effects (myocyte disarray, ECM fibrosis, altered protein phosphorylation levels, etc.) also affect the performance of the myocardium, making it very difficult to isolate the primary effect of the mutation. Our studies on Tm have demonstrated that: (1) Tm positively enhances the hydrophobic interaction between actin and myosin in the "closed state", which in turn enhances the isometric tension; (2) Tm's seven periodical repeats carry distinct functions, with the 3rd period being essential for the tension enhancement; (3) Tm mutants lead to HCM by impairing the relaxation on one hand, and lead to DCM by over inhibition of the AM interaction on the other hand. Ca(2+) sensitivity is affected by inorganic phosphate, ionic strength, and phosphorylation of constituent proteins; hence it may not be the primary cause of the pathogenesis. Here, we review our current knowledge regarding Tm's effect on the actomyosin interaction and the early molecular pathogenesis of Tm mutation related to HCM, DCM, and LVNC.
Figures

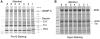

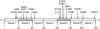
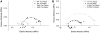
References
-
- Andersen PS, Havndrup O, Hougs L, Sorensen KM, Jensen M, Larsen LA, Hedley P, Thomsen AR, Moolman-Smook J, Christiansen M, Bundgaard H. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Hum Mutat. 2009;30(3):363–370. doi: 10.1002/humu.20862. - DOI - PubMed
-
- Barron JT. Hypertrophic cardiomyopathy. Curr Treat Options Cardiovasc Med. 1999;1:277–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

