Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential
- PMID: 23700507
- PMCID: PMC3661118
- DOI: 10.4161/adip.18951
Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential
Abstract
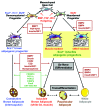
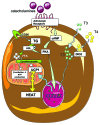
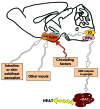
Obesity is currently a global pandemic, and is associated with increased mortality and co-morbidities including many metabolic diseases. Obesity is characterized by an increase in adipose mass due to increased energy intake, decreased energy expenditure, or both. While white adipose tissue is specialized for energy storage, brown adipose tissue has a high concentration of mitochondria and uniquely expresses uncoupling protein 1, enabling it to be specialized for energy expenditure and thermogenesis. Although brown fat was once considered only necessary in babies, recent morphological and imaging studies have provided evidence that, contrary to prior belief, this tissue is present and active in adult humans. In recent years, the topic of brown adipose tissue has been reinvigorated with many new studies regarding brown adipose tissue differentiation, function and therapeutic promise. This review summarizes the recent advances, discusses the emerging questions and offers perspective on the potential therapeutic applications targeting this tissue.
Keywords: BATokine; UCP1; adipogenesis; brown adipose tissue; browning; mitochondria; thermogenesis.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
