Adipose lineage specification of bone marrow-derived myeloid cells
- PMID: 23700536
- PMCID: PMC3609111
- DOI: 10.4161/adip.21496
Adipose lineage specification of bone marrow-derived myeloid cells
Abstract
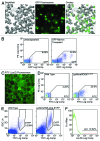
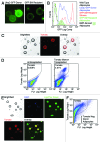
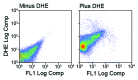
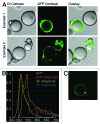
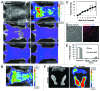
We have reported the production of white adipocytes in adipose tissue from hematopoietic progenitors arising from bone marrow. However, technical challenges have hindered detection of this adipocyte population by certain other laboratories. These disparate results highlight the need for sensitive and definitive techniques to identify bone marrow progenitor (BMP)-derived adipocytes. In these studies we exploited new models and methods to enhance detection of this adipocyte population. Here we showed that confocal microscopy with spectrum acquisition could effectively identify green fluorescent protein (GFP) positive BMP-derived adipocytes by matching their fluorescence spectrum to that of native GFP. Likewise, imaging flow cytometry made it possible to visualize intact unilocular and multilocular GFP-positive BMP-derived adipocytes and distinguished them from non-fluorescent adipocytes and cell debris in the cytometer flow stream. We also devised a strategy to detect marker genes in flow-enriched adipocytes from which stromal cells were excluded. This technique also proved to be an efficient means for detecting genetically labeled adipocytes and should be applicable to models in which marker gene expression is low or absent. Finally, in vivo imaging of mice transplanted with BM from adipocyte-targeted luciferase donors showed a time-dependent increase in luciferase activity, with the bulk of luciferase activity confined to adipocytes rather than stromal cells. These results confirmed and extended our previous reports and provided proof-of-principle for sensitive techniques and models for detection and study of these unique cells.
Keywords: adipocyte; bone marrow; confocal microscopy; imaging flow cytometry; in vivo imaging; progenitor cell; spectral analysis.
Figures


Similar articles
-
Hematopoietic stem cells produce intermediate lineage adipocyte progenitors that simultaneously express both myeloid and mesenchymal lineage markers in adipose tissue.Adipocyte. 2021 Dec;10(1):394-407. doi: 10.1080/21623945.2021.1957290. Adipocyte. 2021. PMID: 34404315 Free PMC article.
-
Serially Transplanted Nonpericytic CD146(-) Adipose Stromal/Stem Cells in Silk Bioscaffolds Regenerate Adipose Tissue In Vivo.Stem Cells. 2016 Apr;34(4):1097-111. doi: 10.1002/stem.2325. Epub 2016 Mar 9. Stem Cells. 2016. PMID: 26865460 Free PMC article.
-
Differentiation of bone marrow-derived cells toward thermogenic adipocytes in white adipose tissue induced by the β3 adrenergic stimulation.FASEB J. 2019 Apr;33(4):5196-5207. doi: 10.1096/fj.201801757RR. Epub 2019 Jan 9. FASEB J. 2019. PMID: 30624970
-
Bone marrow adipose cells - cellular interactions and changes with obesity.J Cell Sci. 2020 Mar 6;133(5):jcs238394. doi: 10.1242/jcs.238394. J Cell Sci. 2020. PMID: 32144195 Review.
-
Bone marrow adipocytes.Adipocyte. 2017 Jul 3;6(3):193-204. doi: 10.1080/21623945.2017.1367881. Epub 2017 Aug 24. Adipocyte. 2017. PMID: 28872979 Free PMC article. Review.
Cited by
-
Intermuscular adipose tissue in metabolic disease.Nat Rev Endocrinol. 2023 May;19(5):285-298. doi: 10.1038/s41574-022-00784-2. Epub 2022 Dec 23. Nat Rev Endocrinol. 2023. PMID: 36564490 Review.
-
Weighing in on adipocyte precursors.Cell Metab. 2014 Jan 7;19(1):8-20. doi: 10.1016/j.cmet.2013.10.003. Epub 2013 Nov 14. Cell Metab. 2014. PMID: 24239569 Free PMC article. Review.
-
Breast Cancer Endocrine Therapy Promotes Weight Gain With Distinct Adipose Tissue Effects in Lean and Obese Female Mice.Endocrinology. 2021 Nov 1;162(11):bqab174. doi: 10.1210/endocr/bqab174. Endocrinology. 2021. PMID: 34410380 Free PMC article.
-
MarrowCellDLD: a microfluidic method for label-free retrieval of fragile bone marrow-derived cells.Sci Rep. 2023 Dec 18;13(1):22462. doi: 10.1038/s41598-023-47978-w. Sci Rep. 2023. PMID: 38105340 Free PMC article.
-
Bone-Fat Interaction.Endocrinol Metab Clin North Am. 2017 Mar;46(1):41-50. doi: 10.1016/j.ecl.2016.09.004. Epub 2016 Nov 24. Endocrinol Metab Clin North Am. 2017. PMID: 28131135 Free PMC article. Review.
References
-
- Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–4. - PubMed
-
- Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
