Effects of water chemistry on aggregation and soil adsorption of silver nanoparticles
- PMID: 23700566
- PMCID: PMC3657714
- DOI: 10.5620/eht.2013.28.e2013006
Effects of water chemistry on aggregation and soil adsorption of silver nanoparticles
Abstract
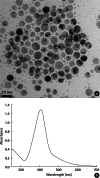
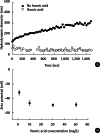
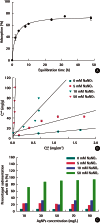
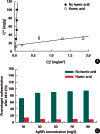
Objectives: In this study, we investigated the influence of ionic strength and natural organic matter (NOM) on aggregation and soil adsorption of citrate-coated silver nanoparticles (AgNPs).
Methods: Time-resolved dynamic light scattering measurements and batch adsorption experiments were used to study their aggregation and soil adsorption behaviors, respectively.
Results: The aggregation rate of AgNPs increased with increasing ionic strength and decreasing NOM concentration. At higher ionic strength, the AgNPs were unstable, and thus tended to be adsorbed to the soil, while increased NOM concentration hindered soil adsorption. To understand the varying behaviors of AgNPs depending on the environmental factors, particle zeta potentials were also measured as a function of ionic strength and NOM concentration. The magnitude of particle zeta potential became more negative with decreasing ionic strength and increasing NOM concentration. These results imply that the aggregation and soil adsorption behavior of AgNPs were mainly controlled by electrical double-layer repulsion consistent with the Derjaguin-Landau-Verwey-Overbeek theory.
Conclusions: This study found that the aggregation and soil adsorption behavior of AgNPs are closely associated with environmental factors such as ionic strength and NOM and suggested that assessing the environmental fate and transport of nanoparticles requires a thorough understanding of particle-particle interaction mechanisms.
Keywords: Aggregation; Ionic strength; Natural organic matter; Silver nanoparticles; Soil adsorption.
Conflict of interest statement
The authors have no conflicts of interest with material presented in this paper.
Figures

References
-
- Niemeyer CM. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew Chem Int Ed. 2001;40(22):4128–4158. - PubMed
-
- Hwang IS, Cho J, Hwang JH, Hwang B, Choi H, Lee J, et al. Antimicrobial effects and mechanism(s) of silver nanoparticle. Korean J Microbiol Biotechnol. 2011;39(1):1–8. (Korean)
-
- Whiteley CM, Valle MD, Kevin C, Jones KC, Sweetman AJ. Challenges in assessing the environmental fate and exposure of nano silver. J Phys Conf Ser. 2011 doi: 10.1088/1742-6596/304/1/012070. - DOI
-
- Project on Emerging Nanotechnologies. An inventory of nanotechnology-based consumer products currently on the market. [cited 2011 Nov 17]. Available from: http://www.nanotechproject.org/inventories/consumer/analysis_draft/
-
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42(11):4133–4139. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

