Pathophysiology of coronary collaterals
- PMID: 23701025
- PMCID: PMC3968593
- DOI: 10.2174/1573403x113099990005
Pathophysiology of coronary collaterals
Abstract
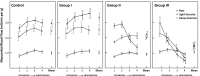
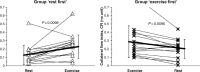
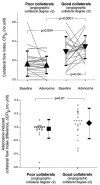
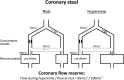
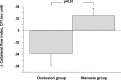
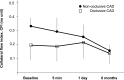
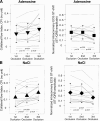
While the existence of structural adaptation of coronary anastomoses is undisputed, the potential of coronary collaterals to be capable of functional adaptation has been questioned. For many years, collateral vessels were thought to be rigid tubes allowing only limited blood flow governed by the pressure gradient across them. This concept was consistent with the notion that although collaterals could provide adequate blood flow to maintain resting levels, they would be unable to increase blood flow sufficiently in situations of increased myocardial oxygen demand. However, more recent studies have demonstrated the capability of the collateral circulation to deliver sufficient blood flow even during exertion or pharmacologic stress. Moreover, it has been shown that increases in collateral flow could be attributed directly to collateral vasomotion. This review summarizes the pathophysiology of the coronary collateral circulation, ie the functional adapation of coronary collaterals to acute alterations in the coronary circulation.
Figures


References
-
- Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. - PubMed
-
- Gregg DE. Coronary Circulation in Health and Disease. Philadelphia PA. Lea & Febiger. 1950
-
- Hoffman JI, Spaan JA. Pressure-flow relations in coronary circulation. Physiol Rev. 1990;70:331–90. - PubMed
-
- Klocke FJ. Coronary blood flow in man. Prog Cardiovasc Dis. 1976;19:117–66. - PubMed
-
- Klocke FJ, Ellis AK. Control of coronary blood flow. Annu Rev Med. 1980;31:489–508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

