Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition
- PMID: 23701408
- PMCID: PMC3806897
- DOI: 10.1021/cb400261h
Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition
Abstract
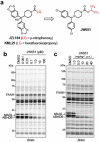
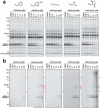
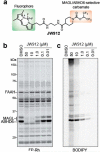
Serine hydrolases are one of the largest and most diverse enzyme classes in Nature. Inhibitors of serine hydrolases are used to treat many diseases, including obesity, diabetes, cognitive dementia, and bacterial and viral infections. Nonetheless, the majority of the 200+ serine hydrolases in mammals still lack selective inhibitors for their functional characterization. We and others have shown that activated carbamates, through covalent reaction with the conserved serine nucleophile of serine hydrolases, can serve as useful inhibitors for members of this enzyme family. The extent to which carbamates, however, cross-react with other protein classes remains mostly unexplored. Here, we address this problem by investigating the proteome-wide reactivity of a diverse set of activated carbamates in vitro and in vivo, using a combination of competitive and click chemistry (CC)-activity-based protein profiling (ABPP). We identify multiple classes of carbamates, including O-aryl, O-hexafluoroisopropyl (HFIP), and O-N-hydroxysuccinimidyl (NHS) carbamates that react selectively with serine hydrolases across entire mouse tissue proteomes in vivo. We exploit the proteome-wide specificity of HFIP carbamates to create in situ imaging probes for the endocannabinoid hydrolases monoacylglycerol lipase (MAGL) and α-β hydrolase-6 (ABHD6). These findings, taken together, designate the carbamate as a privileged reactive group for serine hydrolases that can accommodate diverse structural modifications to produce inhibitors that display exceptional potency and selectivity across the mammalian proteome.
Figures




References
-
- Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin Binding Proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol. Rev. 2006;30:673–691. - PubMed
-
- Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Digestive diseases and sciences. 2007;52:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

