Transfusion requirements in septic shock (TRISS) trial - comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial
- PMID: 23702006
- PMCID: PMC3679866
- DOI: 10.1186/1745-6215-14-150
Transfusion requirements in septic shock (TRISS) trial - comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial
Abstract
Background: Transfusion of red blood cells (RBC) is recommended in septic shock and the majority of these patients receive RBC transfusion in the intensive care unit (ICU). However, benefit and harm of RBCs have not been established in this group of high-risk patients.
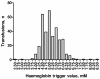
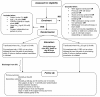
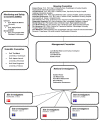
Methods/design: The Transfusion Requirements in Septic Shock (TRISS) trial is a multicenter trial with assessor-blinded outcome assessment, randomising 1,000 patients with septic shock in 30 Scandinavian ICUs to receive transfusion with pre-storage leuko-depleted RBC suspended in saline-adenine-glucose and mannitol (SAGM) at haemoglobin level (Hb) of 7 g/dl or 9 g/dl, stratified by the presence of haematological malignancy and centre. The primary outcome measure is 90-day mortality. Secondary outcome measures are organ failure, ischaemic events, severe adverse reactions (SARs: anaphylactic reaction, acute haemolytic reaction and transfusion-related circulatory overload, and acute lung injury) and mortality at 28 days, 6 months and 1 year.The sample size will enable us to detect a 9% absolute difference in 90-day mortality assuming a 45% event rate with a type 1 error rate of 5% and power of 80%. An interim analysis will be performed after 500 patients, and the Data Monitoring and Safety Committee will recommend the trial be stopped if a group difference in 90-day mortality with P ≤0.001 is present at this point.
Discussion: The TRISS trial may bridge the gap between clinical practice and the lack of efficacy and safety data on RBC transfusion in septic shock patients. The effect of restrictive versus liberal RBC transfusion strategy on mortality, organ failure, ischaemic events and SARs will be evaluated.
Trial registration: ClinicalTrials.gov NCT01485315.
Figures
References
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent J-L, Moreno R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

