Distribution and function of sodium channel subtypes in human atrial myocardium
- PMID: 23702286
- PMCID: PMC3906922
- DOI: 10.1016/j.yjmcc.2013.05.006
Distribution and function of sodium channel subtypes in human atrial myocardium
Abstract
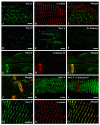
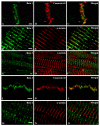
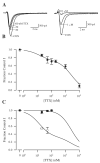
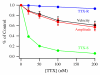
Voltage-gated sodium channels composed of a pore-forming α subunit and auxiliary β subunits are responsible for the upstroke of the action potential in cardiac muscle. However, their localization and expression patterns in human myocardium have not yet been clearly defined. We used immunohistochemical methods to define the level of expression and the subcellular localization of sodium channel α and β subunits in human atrial myocytes. Nav1.2 channels are located in highest density at intercalated disks where β1 and β3 subunits are also expressed. Nav1.4 and the predominant Nav1.5 channels are located in a striated pattern on the cell surface at the z-lines together with β2 subunits. Nav1.1, Nav1.3, and Nav1.6 channels are located in scattered puncta on the cell surface in a pattern similar to β3 and β4 subunits. Nav1.5 comprised approximately 88% of the total sodium channel staining, as assessed by quantitative immunohistochemistry. Functional studies using whole cell patch-clamp recording and measurements of contractility in human atrial cells and tissue showed that TTX-sensitive (non-Nav1.5) α subunit isoforms account for up to 27% of total sodium current in human atrium and are required for maximal contractility. Overall, our results show that multiple sodium channel α and β subunits are differentially localized in subcellular compartments in human atrial myocytes, suggesting that they play distinct roles in initiation and conduction of the action potential and in excitation-contraction coupling. TTX-sensitive sodium channel isoforms, even though expressed at low levels relative to TTX-sensitive Nav1.5, contribute substantially to total cardiac sodium current and are required for normal contractility. This article is part of a Special Issue entitled "Na(+) Regulation in Cardiac Myocytes".
Keywords: Contractility; Immunocytochemistry; Myocardium; Sodium channels.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Clancy CE, Kass RS. Inherited and acquired vulnerability to ventricular arrhythmias: cardiac Na+ and K+ channels. Physiol Rev. 2005;85:33–47. - PubMed
-
- McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E, et al. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–7. - PubMed
-
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
