Glycobiology of cell death: when glycans and lectins govern cell fate
- PMID: 23703323
- PMCID: PMC3705604
- DOI: 10.1038/cdd.2013.50
Glycobiology of cell death: when glycans and lectins govern cell fate
Abstract
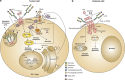
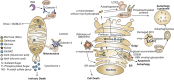
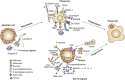
Although one typically thinks of carbohydrates as associated with cell growth and viability, glycosylation also has an integral role in many processes leading to cell death. Glycans, either alone or complexed with glycan-binding proteins, can deliver intracellular signals or control extracellular processes that promote initiation, execution and resolution of cell death programs. Herein, we review the role of glycans and glycan-binding proteins as essential components of the cell death machinery during physiologic and pathologic settings.
Figures
Similar articles
-
Glycobiology of cellular expiry: Decrypting the role of glycan-lectin regulatory complex and therapeutic strategies focusing on cancer.Biochem Pharmacol. 2023 Jan;207:115367. doi: 10.1016/j.bcp.2022.115367. Epub 2022 Dec 5. Biochem Pharmacol. 2023. PMID: 36481348 Review.
-
Glycobiology of the ocular surface: mucins and lectins.Jpn J Ophthalmol. 2013 Mar;57(2):150-5. doi: 10.1007/s10384-012-0228-2. Epub 2013 Jan 17. Jpn J Ophthalmol. 2013. PMID: 23325272 Free PMC article. Review.
-
"Stuck on sugars - how carbohydrates regulate cell adhesion, recognition, and signaling".Glycoconj J. 2019 Aug;36(4):241-257. doi: 10.1007/s10719-019-09876-0. Epub 2019 Jul 2. Glycoconj J. 2019. PMID: 31267247 Free PMC article.
-
Glycobiology of immune responses.Ann N Y Acad Sci. 2012 Apr;1253:1-15. doi: 10.1111/j.1749-6632.2012.06492.x. Ann N Y Acad Sci. 2012. PMID: 22524422 Free PMC article. Review.
-
Glycobiology in the cytosol: the bitter side of a sweet world.Biochim Biophys Acta. 2009 Feb;1790(2):81-94. doi: 10.1016/j.bbagen.2008.09.009. Epub 2008 Oct 8. Biochim Biophys Acta. 2009. PMID: 18952151 Review.
Cited by
-
Harnessing benefit from targeting tumor associated carbohydrate antigens.Hum Vaccin Immunother. 2017 Feb;13(2):323-331. doi: 10.1080/21645515.2017.1264789. Hum Vaccin Immunother. 2017. PMID: 27929800 Free PMC article. Review.
-
Hallmarks of glycosylation in cancer.Oncotarget. 2016 Jun 7;7(23):35478-89. doi: 10.18632/oncotarget.8155. Oncotarget. 2016. PMID: 27007155 Free PMC article. Review.
-
Multi-targeted therapy resistance via drug-induced secretome fucosylation.Elife. 2023 Mar 24;12:e75191. doi: 10.7554/eLife.75191. Elife. 2023. PMID: 36961502 Free PMC article.
-
Galectin-9 in Gastroenterological Cancer.Int J Mol Sci. 2023 Mar 24;24(7):6174. doi: 10.3390/ijms24076174. Int J Mol Sci. 2023. PMID: 37047155 Free PMC article. Review.
-
Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018.Cell Death Differ. 2018 Mar;25(3):486-541. doi: 10.1038/s41418-017-0012-4. Epub 2018 Jan 23. Cell Death Differ. 2018. PMID: 29362479 Free PMC article. Review.
References
-
- Lockshin RA, Williams CM. Programmed cell death-I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J Insect Physiol. 1965;11:123–133. - PubMed
-
- Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

