TORC2 is required to maintain genome stability during S phase in fission yeast
- PMID: 23703609
- PMCID: PMC3707671
- DOI: 10.1074/jbc.M113.464974
TORC2 is required to maintain genome stability during S phase in fission yeast
Abstract
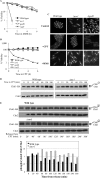
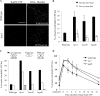
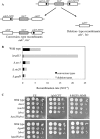
DNA damage can occur due to environmental insults or intrinsic metabolic processes and is a major threat to genome stability. The DNA damage response is composed of a series of well coordinated cellular processes that include activation of the DNA damage checkpoint, transient cell cycle arrest, DNA damage repair, and reentry into the cell cycle. Here we demonstrate that mutant cells defective for TOR complex 2 (TORC2) or the downstream AGC-like kinase, Gad8, are highly sensitive to chronic replication stress but are insensitive to ionizing radiation. We show that in response to replication stress, TORC2 is dispensable for Chk1-mediated cell cycle arrest but is required for the return to cell cycle progression. Rad52 is a DNA repair and recombination protein that forms foci at DNA damage sites and stalled replication forks. TORC2 mutant cells show increased spontaneous nuclear Rad52 foci, particularly during S phase, suggesting that TORC2 protects cells from DNA damage that occurs during normal DNA replication. Consistently, the viability of TORC2-Gad8 mutant cells is dependent on the presence of the homologous recombination pathway and other proteins that are required for replication restart following fork replication stalling. Our findings indicate that TORC2 is required for genome integrity. This may be relevant for the growing amount of evidence implicating TORC2 in cancer development.
Keywords: DNA Damage Response; Fission Yeast; Molecular Cell Biology; Rapamycin; S. pombe; Signal Transduction; Stress; TORC2; Yeast Genetics.
Figures



References
-
- Dazert E., Hall M. N. (2011) mTOR signaling in disease. Curr. Opin. Cell Biol. 23, 744–755 - PubMed
-
- Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 - PubMed
-
- Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

