Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice
- PMID: 23703657
- PMCID: PMC3758784
- DOI: 10.1002/hep.26511
Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice
Abstract
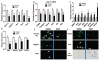
Liver repair involves phenotypic changes in hepatic stellate cells (HSCs) and reactivation of morphogenic signaling pathways that modulate epithelial-to-mesenchymal/mesenchymal-to-epithelial transitions, such as Notch and Hedgehog (Hh). Hh stimulates HSCs to become myofibroblasts (MFs). Recent lineage tracing studies in adult mice with injured livers showed that some MFs became multipotent progenitors to regenerate hepatocytes, cholangiocytes, and HSCs. We studied primary HSC cultures and two different animal models of fibrosis to evaluate the hypothesis that activating the Notch pathway in HSCs stimulates them to become (and remain) MFs through a mechanism that involves an epithelial-to-mesenchymal-like transition and requires cross-talk with the canonical Hh pathway. We found that when cultured HSCs transitioned into MFs, they activated Hh signaling, underwent an epithelial-to-mesenchymal-like transition, and increased Notch signaling. Blocking Notch signaling in MFs/HSCs suppressed Hh activity and caused a mesenchymal-to-epithelial-like transition. Inhibiting the Hh pathway suppressed Notch signaling and also induced a mesenchymal-to-epithelial-like transition. Manipulating Hh and Notch signaling in a mouse multipotent progenitor cell line evoked similar responses. In mice, liver injury increased Notch activity in MFs and Hh-responsive MF progeny (i.e., HSCs and ductular cells). Conditionally disrupting Hh signaling in MFs of bile-duct-ligated mice inhibited Notch signaling and blocked accumulation of both MF and ductular cells.
Conclusions: The Notch and Hedgehog pathways interact to control the fate of key cell types involved in adult liver repair by modulating epithelial-to-mesenchymal-like/mesenchymal-to-epithelial-like transitions.
© 2013 by the American Association for the Study of Liver Diseases.
Figures




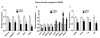

References
-
- Roskams T. Relationships among stellate cell activation, progenitor cells, and hepatic regeneration. Clin Liver Dis. 2008;12:853–860. ix. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
