Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 ± sorafenib
- PMID: 23703789
- PMCID: PMC5097874
- DOI: 10.1002/hep.26487
Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 ± sorafenib
Abstract
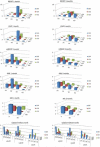
The aim of this study was to compare radiological and pathological changes and test the adjunct efficacy of Sorafenib to Y90 as a bridge to transplantation in hepatocellular carcinoma (HCC). 15 patients with 16 HCC lesions were randomized to Y90 without (Group A, n = 9) or with Sorafenib (Group B, n = 7). Size (WHO, RECIST), enhancement (EASL, mRECIST) and diffusion-weighted imaging criteria (apparent diffusion coefficient, ADC) measurements were obtained at baseline, then at 1 and every 3 months after treatment until transplantation. Percentage necrosis in explanted tumors was correlated with imaging findings. 100%, 50%-99% and <50% pathological necrosis was observed in 6 (67%), 1 (11%), and 2 (22%) tumors in Group A and 3 (42%), 2 (28%), and 2 (28%) in Group B, respectively (P = 0.81). While ADC (P = 0.46) did not change after treatment, WHO (P = 0.06) and RECIST (P = 0.08) response at 1 month failed to reach significance, but significant responses by EASL (P < 0.01/0.03) and mRECIST (P < 0.01/0.03) at 1 and 3 months were observed. Response was equivalent by EASL or mRECIST. No difference in response rates was observed between groups A and B at 1 and 3 months by WHO, RECIST, EASL, mRECIST or ADC measurements. Despite failing to reach significance, smaller baseline size was associated with complete pathological necrosis (CPN) (RECIST: P = 0.07; WHO: P = 0.05). However, a cut-off size of 35 mm was predictive of CPN (P = 0.005). CPN could not be predicted by WHO (P = 0.25 and 0.62), RECIST (P = 0.35 and 0.54), EASL (P = 0.49 and 0.46), mRECIST (P = 0.49 and 0.60) or ADC (P = 0.86 and 0.93).
Conclusion: The adjunct of Sorafenib did not augment radiological or pathological response to Y90 therapy for HCC. Equivalent significant reduction in enhancement at 1 and 3 months by EASL/mRECIST was noted. Neither EASL nor mRECIST could reliably predict CPN.
© 2013 by the American Association for the Study of Liver Diseases.
Figures
References
-
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. - PubMed
-
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. - PubMed
-
- Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, Abecassis M, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49:1185–1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical