Equilibrative nucleoside transporter (ENT)-1-dependent elevation of extracellular adenosine protects the liver during ischemia and reperfusion
- PMID: 23703920
- PMCID: PMC3795856
- DOI: 10.1002/hep.26505
Equilibrative nucleoside transporter (ENT)-1-dependent elevation of extracellular adenosine protects the liver during ischemia and reperfusion
Abstract
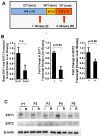
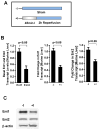
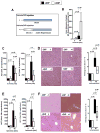
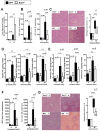
Ischemia and reperfusion-elicited tissue injury contributes to morbidity and mortality of hepatic surgery and during liver transplantation. Previous studies implicated extracellular adenosine signaling in liver protection. Based on the notion that extracellular adenosine signaling is terminated by uptake from the extracellular towards the intracellular compartment by way of equilibrative nucleoside transporters (ENTs), we hypothesized a functional role of ENTs in liver protection from ischemia. During orthotopic liver transplantation in humans, we observed higher expressional levels of ENT1 than ENT2, in conjunction with repression of ENT1 and ENT2 transcript and protein levels following warm ischemia and reperfusion. Treatment with the pharmacologic ENT inhibitor dipyridamole revealed elevations of hepatic adenosine levels and robust liver protection in a murine model of liver ischemia and reperfusion. Studies in gene-targeted mice for Ent1 or Ent2 demonstrated selective protection from liver injury in Ent1(-/-) mice. Treatment with selective adenosine receptor antagonists indicated a contribution of Adora2b receptor signaling in ENT-dependent liver protection.
Conclusion: These findings implicate ENT1 in liver protection from ischemia and reperfusion injury and suggest ENT inhibitors may be of benefit in the prevention or treatment of ischemic liver injury.
© 2013 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures


References
-
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
