Membrane versus soluble isoforms of TNF-α exert opposing effects on tumor growth and survival of tumor-associated myeloid cells
- PMID: 23704210
- PMCID: PMC3702680
- DOI: 10.1158/0008-5472.CAN-13-0002
Membrane versus soluble isoforms of TNF-α exert opposing effects on tumor growth and survival of tumor-associated myeloid cells
Erratum in
- Cancer Res. 2014 Feb 1;74(3):976
Abstract
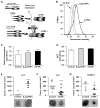
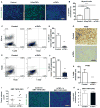
TNF-α, produced by most malignant cells, orchestrates the interplay between malignant cells and myeloid cells, which have been linked to tumor growth and metastasis. Although TNF-α can exist as one of two isoforms, a 26-kDa membrane tethered form (mTNF-α) or a soluble 17-kDa cytokine (sTNF-α), the vast majority of published studies have only investigated the biologic effects of the soluble form. We show for the first time that membrane and soluble isoforms have diametrically opposing effects on both tumor growth and myeloid content. Mouse lung and melanoma tumor lines expressing mTNF-α generated small tumors devoid of monocytes versus respective control lines or lines expressing sTNF-α. The lack of myeloid cells was due to a direct effect of mTNF-α on myeloid survival via induction of cell necrosis by increasing reactive oxygen species. Human non-small cell lung carcinoma expressed varying levels of both soluble and membrane TNF-α, and gene expression patterns favoring mTNF-α were predictive of improved lung cancer survival. These data suggest that there are significant differences in the role of different TNF-α isoforms in tumor progression and the bioavailability of each isoform may distinctly regulate tumor progression. This insight is critical for effective intervention in cancer therapy with the available TNF-α inhibitors, which can block both TNF-α isoforms.
©2013 AACR.
Conflict of interest statement
Figures





References
-
- Bozcuk H, Uslu G, Samur M, et al. Tumour necrosis factor-alpha, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine. 2004;27:58–65. - PubMed
-
- Ferrajoli A, Keating MJ, Manshouri T, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–9. - PubMed
-
- Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

