Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms
- PMID: 23704576
- PMCID: PMC3816976
- DOI: 10.1126/science.1237874
Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms
Abstract
CD8(+) T cell responses focus on a small fraction of pathogen- or vaccine-encoded peptides, and for some pathogens, these restricted recognition hierarchies limit the effectiveness of antipathogen immunity. We found that simian immunodeficiency virus (SIV) protein-expressing rhesus cytomegalovirus (RhCMV) vectors elicit SIV-specific CD8(+) T cells that recognize unusual, diverse, and highly promiscuous epitopes, including dominant responses to epitopes restricted by class II major histocompatibility complex (MHC) molecules. Induction of canonical SIV epitope-specific CD8(+) T cell responses is suppressed by the RhCMV-encoded Rh189 gene (corresponding to human CMV US11), and the promiscuous MHC class I- and class II-restricted CD8(+) T cell responses occur only in the absence of the Rh157.5, Rh157.4, and Rh157.6 (human CMV UL128, UL130, and UL131) genes. Thus, CMV vectors can be genetically programmed to achieve distinct patterns of CD8(+) T cell epitope recognition.
Figures




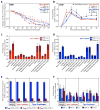
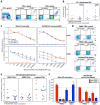

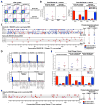
Comment in
-
Immunology. Antigen processing takes a new direction.Science. 2013 May 24;340(6135):937-8. doi: 10.1126/science.1239649. Science. 2013. PMID: 23704564 No abstract available.
-
Programming CMV for vaccine vector design.Nat Biotechnol. 2013 Sep;31(9):811-2. doi: 10.1038/nbt.2688. Nat Biotechnol. 2013. PMID: 24022159 No abstract available.
References
-
- Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823. - PubMed
-
- Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25:533. - PubMed
-
- Irvine K, Bennink J. Factors influencing immunodominance hierarchies in TCD8+-mediated antiviral responses. Expert Rev Clin Immunol. 2006;2:135. - PubMed
-
- Assarsson E, et al. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol. 2007;178:7890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

