Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY
- PMID: 23704674
- PMCID: PMC3661836
- DOI: 10.2337/dc12-2393
Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY
Abstract
Objective: The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) trial demonstrated that combination therapy with metformin plus rosiglitazone provided superior durability of glycemic control compared with metformin alone, with significantly lower treatment failure rates (38.6 vs. 51.7%), and metformin plus lifestyle was intermediate. Herein we describe the temporal changes in measures of β-cell function and insulin sensitivity over a 4-year period among the three treatments.
Research design and methods: TODAY participants (699) were tested periodically with an oral glucose tolerance test to determine insulin sensitivity (1/fasting insulin [1/IF]), insulinogenic index (ΔI(30)/ΔG(30)) or C-peptide index (ΔC(30)/ΔG(30)), and β-cell function relative to insulin sensitivity (oral disposition index [oDI]).
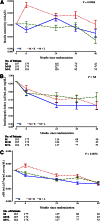
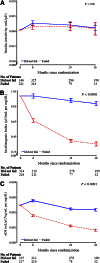
Results: During the first 6 months, metformin plus rosiglitazone exhibited a significantly greater improvement in insulin sensitivity and oDI versus metformin alone and versus metformin plus lifestyle; these improvements were sustained over 48 months of TODAY. Irrespective of treatment, those who failed to maintain glycemic control had significantly lower β-cell function (~50%), higher fasting glucose concentration, and higher HbA1c at randomization compared with those who did not fail.
Conclusions: The beneficial change in insulin sensitivity and the resultant lower burden on β-cell function achieved in the first 6 months with metformin plus rosiglitazone appear to be responsible for its superior glycemic durability over metformin alone and metformin plus lifestyle. However, initial β-cell reserve and HbA1c at randomization are independent predictors of glycemic durability. Therefore, efforts to preserve β-cell function before significant loss occurs and to reduce HbA1c may be beneficial in the treatment of youth with type 2 diabetes.
Trial registration: ClinicalTrials.gov NCT00081328.
Figures
Comment in
-
Comment on: TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749-1757.Diabetes Care. 2013 Dec;36(12):e223. doi: 10.2337/dc13-1426. Diabetes Care. 2013. PMID: 24265392 Free PMC article. No abstract available.
References
-
- Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002;25:89–94 - PubMed
-
- Zeitler P, Epstein L, Grey M, et al. TODAY Study Group Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- M01-RR00125/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- UL1-RR025758/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U01 DK061212/DK/NIDDK NIH HHS/United States
- M01 RR014467/RR/NCRR NIH HHS/United States
- U01-DK-61212/DK/NIDDK NIH HHS/United States
- M01-RR00036/RR/NCRR NIH HHS/United States
- M01 RR000084/RR/NCRR NIH HHS/United States
- M01-RR00043-45/RR/NCRR NIH HHS/United States
- U01-DK-61239/DK/NIDDK NIH HHS/United States
- U01 DK061254/DK/NIDDK NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- UL1-RR024153/RR/NCRR NIH HHS/United States
- U01-DK-61230/DK/NIDDK NIH HHS/United States
- UL1-RR024989/RR/NCRR NIH HHS/United States
- U01 DK061242/DK/NIDDK NIH HHS/United States
- M01-RR01066/RR/NCRR NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- U01-DK-61254/DK/NIDDK NIH HHS/United States
- U01 DK061230/DK/NIDDK NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1-RR024139/RR/NCRR NIH HHS/United States
- UL1-RR025780/RR/NCRR NIH HHS/United States
- UL1-RR024992/RR/NCRR NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- UL1-RR024134/RR/NCRR NIH HHS/United States
- U01-DK-61242/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01-RR00069/RR/NCRR NIH HHS/United States
- M01-RR14467/RR/NCRR NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- U01 DK061239/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- M01-RR00084/RR/NCRR NIH HHS/United States
- UL1 TR000130/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

