Glioma initiating cells form a differentiation niche via the induction of extracellular matrices and integrin αV
- PMID: 23704872
- PMCID: PMC3660593
- DOI: 10.1371/journal.pone.0059558
Glioma initiating cells form a differentiation niche via the induction of extracellular matrices and integrin αV
Abstract
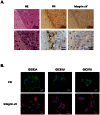
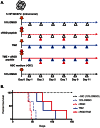
Glioma initiating cells (GICs) are considered responsible for the therapeutic resistance and recurrence of malignant glioma. To clarify the molecular mechanism of GIC maintenance/differentiation, we established GIC clones having the potential to differentiate into malignant gliomas, and subjected to DNA microarray/iTRAQ based integrated proteomics. 21,857 mRNAs and 8,471 proteins were identified and integrated into a gene/protein expression analysis chart. Gene Ontology analysis revealed that the expression of cell adhesion molecules, including integrin subfamilies, such as α2 and αV, and extracellular matrices (ECMs), such as collagen IV (COL4), laminin α2 (LAMA2), and fibronectin 1 (FN), was significantly upregulated during serum-induced GIC differentiation. This differentiation process, accompanied by the upregulation of MAPK as well as glioma specific proteins in GICs, was dramatically accelerated in these ECM (especially FN)-coated dishes. Integrin αV blocking antibody and RGD peptide significantly suppressed early events in GIC differentiation, suggesting that the coupling of ECMs to integrin αV is necessary for GIC differentiation. In addition, the expression of integrin αV and its strong ligand FN was prominently increased in glioblastomas developed from mouse intracranial GIC xenografts. Interestingly, during the initial phase of GIC differentiation, the RGD treatment significantly inhibited GIC proliferation and raised their sensitivity against anti-cancer drug temozolomide (TMZ). We also found that combination treatments of TMZ and RGD inhibit glioma progression and lead the longer survival of mouse intracranial GIC xenograft model. These results indicate that GICs induce/secrete ECMs to develop microenvironments with serum factors, namely differentiation niches that further stimulate GIC differentiation and proliferation via the integrin recognition motif RGD. A combination of RGD treatment with TMZ could have the higher inhibitory potential against the glioma recurrence that may be regulated by the GICs in the differentiation niche. This study provides a new perspective for developing therapeutic strategies against the early onset of GIC-associated glioma.
Conflict of interest statement
Figures




References
-
- Stupp R, Pavlidis N, Jelic S (2005) ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO 16 Suppl 1: i64–65. - PubMed
-
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, et al. (2009) Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 4: 568–580. - PubMed
-
- Rich JN, Bao S (2007) Chemotherapy and cancer stem cells. Cell stem cell 1: 353–355. - PubMed
-
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116: 769–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

