Increased cholesterol content in gammadelta (γδ) T lymphocytes differentially regulates their activation
- PMID: 23704936
- PMCID: PMC3660587
- DOI: 10.1371/journal.pone.0063746
Increased cholesterol content in gammadelta (γδ) T lymphocytes differentially regulates their activation
Abstract
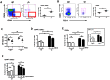
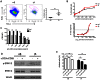
Gammadelta (γδ) T lymphocytes respond quickly upon antigen encounter to produce a cytokine response. In this study, we sought to understand how functions of γδ T cells are differentially regulated compared to αβ T cells. We found that cholesterol, an integral component of the plasma membrane and a regulator of TCR signaling, is increased in γδ T cells compared to αβ T cells, and modulates their function. Higher levels of activation markers, and increased lipid raft content in γδ cells suggest that γδ T cells are more activated. Cholesterol depletion effectively decreased lipid raft formation and activation of γδ T cells, indicating that increased cholesterol content contributes to the hyper-activated phenotype of γδ T cells, possibly through enhanced clustering of TCR signals in lipid rafts. TCR stimulation assays and western blotting revealed that instead of a lower TCR threshold, enhanced TCR signaling through ERK1/2 activation is likely the cause for high cholesterol-induced rapid activation and proliferation in γδ T cells. Our data indicate that cholesterol metabolism is differentially regulated in γδ T cells. The high intracellular cholesterol content leads to enhanced TCR signaling and increases activation and proliferation of γδ T cells.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

