Integrative study of physiological changes associated with bacterial infection in Pacific oyster larvae
- PMID: 23704993
- PMCID: PMC3660371
- DOI: 10.1371/journal.pone.0064534
Integrative study of physiological changes associated with bacterial infection in Pacific oyster larvae
Abstract
Background: Bacterial infections are common in bivalve larvae and can lead to significant mortality, notably in hatcheries. Numerous studies have identified the pathogenic bacteria involved in such mortalities, but physiological changes associated with pathogen exposure at larval stage are still poorly understood. In the present study, we used an integrative approach including physiological, enzymatic, biochemical, and molecular analyses to investigate changes in energy metabolism, lipid remodelling, cellular stress, and immune status of Crassostrea gigas larvae subjected to experimental infection with the pathogenic bacteria Vibrio coralliilyticus.
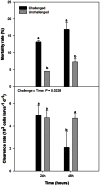
Findings: Our results showed that V. coralliilyticus exposure induced (1) limited but significant increase of larvae mortality compared with controls, (2) declined feeding activity, which resulted in energy status changes (i.e. reserve consumption, β-oxidation, decline of metabolic rate), (3) fatty acid remodeling of polar lipids (changes in phosphatidylinositol and lysophosphatidylcholine composition`, non-methylene-interrupted fatty acids accumulation, lower content of major C20 polyunsaturated fatty acids as well as activation of desaturases, phospholipase and lipoxygenase), (4) activation of antioxidant defenses (catalase, superoxide dismutase, peroxiredoxin) and cytoprotective processes (heat shock protein 70, pernin), and (5) activation of the immune response (non-self recognition, NF-κκ signaling pathway, haematopoiesis, eiconosoids and lysophosphatidyl acid synthesis, inhibitor of metalloproteinase and antimicrobial peptides).
Conclusion: Overall, our results allowed us to propose an integrative view of changes induced by a bacterial infection in Pacific oyster larvae, opening new perspectives on the response of marine bivalve larvae to infections.
Conflict of interest statement
Figures



Similar articles
-
Bacteriophages against Vibrio coralliilyticus and Vibrio tubiashii: Isolation, Characterization, and Remediation of Larval Oyster Mortalities.Appl Environ Microbiol. 2021 Apr 27;87(10):e00008-21. doi: 10.1128/AEM.00008-21. Print 2021 Apr 27. Appl Environ Microbiol. 2021. PMID: 33674441 Free PMC article.
-
Immunomodulatory effects of a probiotic combination treatment to improve the survival of Pacific oyster (Crassostrea gigas) larvae against infection by Vibrio coralliilyticus.Front Immunol. 2024 Apr 8;15:1380089. doi: 10.3389/fimmu.2024.1380089. eCollection 2024. Front Immunol. 2024. PMID: 38650950 Free PMC article.
-
Mortalities of Eastern and Pacific oyster Larvae caused by the pathogens Vibrio coralliilyticus and Vibrio tubiashii.Appl Environ Microbiol. 2015 Jan;81(1):292-7. doi: 10.1128/AEM.02930-14. Epub 2014 Oct 24. Appl Environ Microbiol. 2015. PMID: 25344234 Free PMC article.
-
Application of the bacteriophage pVco-14 to prevent Vibrio coralliilyticus infection in Pacific oyster (Crassostrea gigas) larvae.J Invertebr Pathol. 2019 Oct;167:107244. doi: 10.1016/j.jip.2019.107244. Epub 2019 Sep 11. J Invertebr Pathol. 2019. PMID: 31520593
-
Contrasting Immunomodulatory Effects of Probiotic and Pathogenic Bacteria on Eastern Oyster, Crassostrea Virginica, Larvae.Vaccines (Basel). 2020 Oct 6;8(4):588. doi: 10.3390/vaccines8040588. Vaccines (Basel). 2020. PMID: 33036213 Free PMC article.
Cited by
-
Comparative genomic analysis of Vibrios yields insights into genes associated with virulence towards C. gigas larvae.BMC Genomics. 2020 Aug 31;21(1):599. doi: 10.1186/s12864-020-06980-6. BMC Genomics. 2020. PMID: 32867668 Free PMC article.
-
First Report of Vibrio tubiashii Associated with a Massive Larval Mortality Event in a Commercial Hatchery of Scallop Argopecten purpuratus in Chile.Front Microbiol. 2016 Sep 20;7:1473. doi: 10.3389/fmicb.2016.01473. eCollection 2016. Front Microbiol. 2016. Retraction in: Front Microbiol. 2018 Aug 30;9:2168. doi: 10.3389/fmicb.2018.02168. PMID: 27703450 Free PMC article. Retracted.
-
Larval Geoduck (Panopea generosa) Proteomic Response to Ciliates.Sci Rep. 2020 Apr 8;10(1):6042. doi: 10.1038/s41598-020-63218-x. Sci Rep. 2020. PMID: 32269285 Free PMC article.
-
Lipidomic insights into the immune response and pearl formation in transplanted pearl oyster Pinctada fucata martensii.Front Immunol. 2022 Oct 7;13:1018423. doi: 10.3389/fimmu.2022.1018423. eCollection 2022. Front Immunol. 2022. PMID: 36275716 Free PMC article.
-
Different responses of larval fatty acid profiles to cryopreservation in two commercially important bivalves.Sci Rep. 2024 Oct 19;14(1):24582. doi: 10.1038/s41598-024-76723-0. Sci Rep. 2024. PMID: 39427064 Free PMC article.
References
-
- Gestal C, Roch P, Renault T, Pallavicini A, Paillard C, et al. (2008) Study of diseases and the immune system of bivalves using molecular biology and genomics. Rev. Fish. 16: 133–156.
-
- Song LS, Wang LL, Qiu LM, Zhang HA (2010) Bivalve immunity. In: Soderhall K, editor. Invertebrate Immunity. Berlin: Springer-Verlag Berlin. pp. 44–65.
-
- Fleury E, Huvet A (2012) Microarray analysis highlights immune response of pacific oysters as a determinant of resistance to summer mortality. Mar Biotechnol 14: 203–217. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

