MARCKS protein is phosphorylated and regulates calcium mobilization during human acrosomal exocytosis
- PMID: 23704996
- PMCID: PMC3660367
- DOI: 10.1371/journal.pone.0064551
MARCKS protein is phosphorylated and regulates calcium mobilization during human acrosomal exocytosis
Abstract
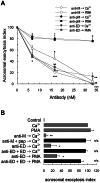
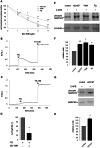
Acrosomal exocytosis is a calcium-regulated exocytosis that can be triggered by PKC activators. The involvement of PKC in acrosomal exocytosis has not been fully elucidated, and it is unknown if MARCKS, the major substrate for PKC, participates in this exocytosis. Here, we report that MARCKS is expressed in human spermatozoa and localizes to the sperm head and the tail. Calcium- and phorbol ester-triggered acrosomal exocytosis in permeabilized sperm was abrogated by different anti-MARCKS antibodies raised against two different domains, indicating that the protein participates in acrosomal exocytosis. Interestingly, an anti-phosphorylated MARCKS antibody was not able to inhibit secretion. Similar results were obtained using recombinant proteins and phospho-mutants of MARCKS effector domain (ED), indicating that phosphorylation regulates MARCKS function in acrosomal exocytosis. It is known that unphosphorylated MARCKS sequesters PIP2. This phospholipid is the precursor for IP3, which in turn triggers release of calcium from the acrosome during acrosomal exocytosis. We found that PIP2 and adenophostin, a potent IP3-receptor agonist, rescued MARCKS inhibition in permeabilized sperm, suggesting that MARCKS inhibits acrosomal exocytosis by sequestering PIP2 and, indirectly, MARCKS regulates the intracellular calcium mobilization. In non-permeabilized sperm, a permeable peptide of MARCKS ED also inhibited acrosomal exocytosis when stimulated by a natural agonist such as progesterone, and pharmacological inducers such as calcium ionophore and phorbol ester. The preincubation of human sperm with the permeable MARCKS ED abolished the increase in calcium levels caused by progesterone, demonstrating that MARCKS regulates calcium mobilization. In addition, the phosphorylation of MARCKS increased during acrosomal exocytosis stimulated by the same activators. Altogether, these results show that MARCKS is a negative modulator of the acrosomal exocytosis, probably by sequestering PIP2, and that it is phosphorylated during acrosomal exocytosis.
Conflict of interest statement
Figures




References
-
- Yanagimachi R (1994) Mammalian fertilization. In: Knobil, E., Neill, J.D. (Eds.), The Physiology of Reproduction. Raven Press. In. pp. 189–281.
-
- Breitbart H (2002) Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol Cell Endocrinol 187: 139–144. - PubMed
-
- Sistina Y, Lin M, Mate KE, Rodger JC (1993) Induction of the marsupial sperm acrosome reaction in vitro by treatment with diacylglycerols. J Reprod Fertil 99: 335–341. - PubMed
-
- De Jonge CJ, Han HL, Mack SR, Zaneveld LJ (1991) Effect of phorbol diesters, synthetic diacylglycerols, and a protein kinase C inhibitor on the human sperm acrosome reaction. J Androl 12: 62–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

