Neurological characterization of mice deficient in GSK3α highlight pleiotropic physiological functions in cognition and pathological activity as Tau kinase
- PMID: 23705847
- PMCID: PMC3671145
- DOI: 10.1186/1756-6606-6-27
Neurological characterization of mice deficient in GSK3α highlight pleiotropic physiological functions in cognition and pathological activity as Tau kinase
Abstract
Background: GSK3β is involved in a wide range of physiological functions, and is presumed to act in the pathogenesis of neurological diseases, from bipolar disorder to Alzheimer's disease (AD). In contrast, the GSK3α isozyme remained largely ignored with respect to both aspects.
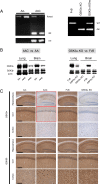
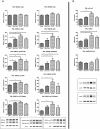
Results: We generated and characterized two mouse strains with neuron-specific or with total GSK3α deficiency. Behavioral and electrophysiological analysis demonstrated the physiological importance of neuronal GSK3α, with GSK3β not compensating for impaired cognition and reduced LTP. Interestingly, the passive inhibitory avoidance task proved to modulate the phosphorylation status of both GSK3 isozymes in wild-type mice, further implying both to function in cognition. Moreover, GSK3α contributed to the neuronal architecture of the hippocampal CA1 sub-region that is most vulnerable in AD. Consequently, practically all parameters and characteristics indicated that both GSK3 isoforms were regulated independently, but that they acted on the same physiological functions in learning and memory, in mobility and in behavior.
Conclusions: GSK3α proved to be regulated independently from GSK3β, and to exert non-redundant physiological neurological functions in general behavior and in cognition. Moreover, GSK3α contributes to the pathological phosphorylation of protein Tau.
Figures








Similar articles
-
GSK3β isoform-selective regulation of depression, memory and hippocampal cell proliferation.Genes Brain Behav. 2016 Mar;15(3):348-55. doi: 10.1111/gbb.12283. Epub 2016 Feb 12. Genes Brain Behav. 2016. PMID: 26749572 Free PMC article.
-
Dynamic range of GSK3α not GSK3β is essential for bidirectional synaptic plasticity at hippocampal CA3-CA1 synapses.Hippocampus. 2014 Dec;24(12):1413-6. doi: 10.1002/hipo.22362. Epub 2014 Oct 1. Hippocampus. 2014. PMID: 25208523 Free PMC article.
-
GSK3α, not GSK3β, drives hippocampal NMDAR-dependent LTD via tau-mediated spine anchoring.EMBO J. 2021 Jan 15;40(2):e105513. doi: 10.15252/embj.2020105513. Epub 2020 Nov 16. EMBO J. 2021. PMID: 33197065 Free PMC article.
-
GSK3 in Alzheimer's disease: mind the isoforms.J Alzheimers Dis. 2014;39(4):707-10. doi: 10.3233/JAD-131661. J Alzheimers Dis. 2014. PMID: 24254703 Free PMC article. Review.
-
GSK3 and tau: two convergence points in Alzheimer's disease.J Alzheimers Dis. 2013;33 Suppl 1:S141-4. doi: 10.3233/JAD-2012-129025. J Alzheimers Dis. 2013. PMID: 22710914 Review.
Cited by
-
Tau Acts in Concert With Kinase/Phosphatase Underlying Synaptic Dysfunction.Front Aging Neurosci. 2022 May 27;14:908881. doi: 10.3389/fnagi.2022.908881. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35711910 Free PMC article. Review.
-
Differential role of bovine serum albumin and HCO3- in the regulation of GSK3 alpha during mouse sperm capacitation.Mol Hum Reprod. 2024 Feb 29;30(3):gaae007. doi: 10.1093/molehr/gaae007. Mol Hum Reprod. 2024. PMID: 38341666 Free PMC article.
-
Loss of glycogen synthase kinase 3 isoforms during murine oocyte growth induces offspring cardiac dysfunction.Biol Reprod. 2015 May;92(5):127. doi: 10.1095/biolreprod.115.128181. Epub 2015 Apr 1. Biol Reprod. 2015. PMID: 25833158 Free PMC article.
-
Structural Plasticity of Dendritic Spines Requires GSK3α and GSK3β.PLoS One. 2015 Jul 24;10(7):e0134018. doi: 10.1371/journal.pone.0134018. eCollection 2015. PLoS One. 2015. PMID: 26207897 Free PMC article.
-
GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration.Neural Plast. 2019 May 2;2019:4209475. doi: 10.1155/2019/4209475. eCollection 2019. Neural Plast. 2019. PMID: 31191636 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

