The interaction of celecoxib with MDR transporters enhances the activity of mitomycin C in a bladder cancer cell line
- PMID: 23705854
- PMCID: PMC3669624
- DOI: 10.1186/1476-4598-12-47
The interaction of celecoxib with MDR transporters enhances the activity of mitomycin C in a bladder cancer cell line
Abstract
Background: An in vitro model was developed to understand if celecoxib could synergize with Mitomycin C (MMC), commonly used for the prevention of non-muscle invasive bladder cancer recurrence, and eventually elucidate if the mechanism of interaction involves multi drug resistance (MDR) transporters.
Methods: UMUC-3, a non COX-2 expressing bladder cancer cell line, and UMUC-3-CX, a COX-2 overexpressing transfectant, as well as 5637, a COX-2 overexpressing cell line, and 5637si-CX, a non COX-2 expressing silenced 5637 cell line, were used in the present study. The expression of COX-2 and MDR pumps (P-gp, MDR-1 and BCRP) was explored through western blot. The anti-proliferative effect of celecoxib and MMC was studied with MTT test. Three biological permeability assays (Drug Transport Experiment, Substrate Transporter Inhibition, and ATP cell depletion) were combined to study the interaction between MDR transporters and celecoxib. Finally, the ability of celecoxib to restore MMC cell accumulation was investigated.
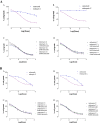
Results: The anti-proliferative effect of celecoxib and MMC were investigated alone and in co-administration, in UMUC-3, UMUC-3-CX, 5637 and 5637si-CX cells. When administered alone, the effect of MMC was 8-fold greater in UMUC-3. However, co-administration of 1 μM, 5 μM, and 10 μM celecoxib and MMC caused a 2,3-fold cytotoxicity increase in UMUC-3-CX cell only. MMC cytotoxicity was not affected by celecoxib co-administration either in 5637, or in 5637si-CX cells. As a result of all finding from the permeability experiments, celecoxib was classified as P-gp unambiguous substrate: celecoxib is transported by MDR pumps and interferes with the efflux of MMC. Importantly, among all transporters, BCRP was only overexpressed in UMUC-3-CX cells, but not in 5637 and 5637si-CX.
Conclusions: The UMUC-3-CX cell line resembles a more aggressive phenotype with a lower response to MMC compared to the wt counterpart. However, the administration of celecoxib in combination to MMC causes a significant and dose dependent gain of the anti-proliferative activity. This finding may be the result of a direct interaction between celecoxib and MDR transporters. Indeed, BCRP is overexpressed in UMUC-3-CX, but not in UMUC-3, 5637, and 5637si-CX, in which celecoxib is ineffective.
Figures




References
-
- Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Donald LD, Newling WW. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. - DOI - PubMed
-
- Pawinski A, Sylvester R, Kurth KH, Bouffioux C, van der der Meijden A, Parmar MK, Bijnens L. A combined analysis of european organization for research and treatment of cancer, and medical research council randomized clinical trials for the prophylactic treatment of TaT1 bladder cancer. European organization for research and treatment of cancer genitourinary tract cancer cooperative group and the medical research council working part on superficial bladder cancer. J Urol. 1996;156:1934–1941. doi: 10.1016/S0022-5347(01)65396-5. - DOI - PubMed
-
- Klan R, Loy V, Huland H. Residual tumor discovered in routine second transurethral resection in patients with stage T1 transitional cell carcinoma of the bladder. J Urol. 1991;146:316–318. - PubMed
-
- Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol. 2006;175:1641–1644. doi: 10.1016/S0022-5347(05)01002-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

