Genome-scale metabolic network guided engineering of Streptomyces tsukubaensis for FK506 production improvement
- PMID: 23705993
- PMCID: PMC3680238
- DOI: 10.1186/1475-2859-12-52
Genome-scale metabolic network guided engineering of Streptomyces tsukubaensis for FK506 production improvement
Abstract
Background: FK506 is an important immunosuppressant, which can be produced by Streptomyces tsukubaensis. However, the production capacity of the strain is very low. Hereby, a computational guided engineering approach was proposed in order to improve the intracellular precursor and cofactor availability of FK506 in S. tsukubaensis.
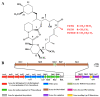
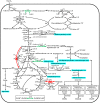
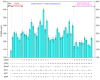
Results: First, a genome-scale metabolic model of S. tsukubaensis was constructed based on its annotated genome and biochemical information. Subsequently, several potential genetic targets (knockout or overexpression) that guaranteed an improved yield of FK506 were identified by the recently developed methodology. To validate the model predictions, each target gene was manipulated in the parent strain D852, respectively. All the engineered strains showed a higher FK506 production, compared with D852. Furthermore, the combined effect of the genetic modifications was evaluated. Results showed that the strain HT-ΔGDH-DAZ with gdhA-deletion and dahp-, accA2-, zwf2-overexpression enhanced FK506 concentration up to 398.9 mg/L, compared with 143.5 mg/L of the parent strain D852. Finally, fed-batch fermentations of HT-ΔGDH-DAZ were carried out, which led to the FK506 production of 435.9 mg/L, 1.47-fold higher than the parent strain D852 (158.7 mg/L).
Conclusions: Results confirmed that the promising targets led to an increase in FK506 titer. The present work is the first attempt to engineer the primary precursor pathways to improve FK506 production in S. tsukubaensis with genome-scale metabolic network guided metabolic engineering. The relationship between model prediction and experimental results demonstrates the rationality and validity of this approach for target identification. This strategy can also be applied to the improvement of other important secondary metabolites.
Figures





References
-
- Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

