SHILO, a novel dual imaging approach for simultaneous HI-/LOw temporal (Low-/Hi-spatial) resolution imaging for vascular dynamic contrast enhanced cardiovascular magnetic resonance: numerical simulations and feasibility in the carotid arteries
- PMID: 23706156
- PMCID: PMC3668185
- DOI: 10.1186/1532-429X-15-42
SHILO, a novel dual imaging approach for simultaneous HI-/LOw temporal (Low-/Hi-spatial) resolution imaging for vascular dynamic contrast enhanced cardiovascular magnetic resonance: numerical simulations and feasibility in the carotid arteries
Abstract
Background: Dynamic contrast enhanced (DCE) cardiovascular magnetic resonance (CMR) is increasingly used to quantify microvessels and permeability in atherosclerosis. Accurate quantification depends on reliable sampling of both vessel wall (VW) uptake and contrast agent dynamic in the blood plasma (the so called arterial input function, AIF). This poses specific challenges in terms of spatial/temporal resolution and matched dynamic MR signal range, which are suboptimal in current vascular DCE-CMR protocols. In this study we describe a novel dual-imaging approach, which allows acquiring simultaneously AIF and VW images using different spatial/temporal resolution and optimizes imaging parameters for the two compartments. We refer to this new acquisition as SHILO, Simultaneous HI-/LOw-temporal (low-/hi-spatial) resolution DCE-imaging.
Methods: In SHILO, the acquisition of low spatial resolution single-shot AIF images is interleaved with segments of higher spatial resolution images of the VW. This allows sampling the AIF and VW with different spatial/temporal resolution and acquisition parameters, at independent spatial locations. We show the adequacy of this temporal sampling scheme by using numerical simulations. Following, we validate the MR signal of SHILO against a standard 2D spoiled gradient recalled echo (SPGR) acquisition with in vitro and in vivo experiments. Finally, we show feasibility of using SHILO imaging in subjects with carotid atherosclerosis.
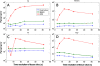
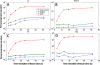
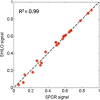
Results: Our simulations confirmed the superiority of the SHILO temporal sampling scheme over conventional strategies that sample AIF and tissue curves at the same time resolution. Both the median relative errors and standard deviation of absolute parameter values were lower for the SHILO than for conventional sampling schemes. We showed equivalency of the SHILO signal and conventional 2D SPGR imaging, using both in vitro phantom experiments (R2 =0.99) and in vivo acquisitions (R2 =0.95). Finally, we showed feasibility of using the newly developed SHILO sequence to acquire DCE-CMR data in subjects with carotid atherosclerosis to calculate plaque perfusion indices.
Conclusions: We successfully demonstrate the feasibility of using the newly developed SHILO dual-imaging technique for simultaneous AIF and VW imaging in DCE-CMR of atherosclerosis. Our initial results are promising and warrant further investigation of this technique in wider studies measuring kinetic parameters of plaque neovascularization with validation against gold standard techniques.
Figures





References
-
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–8. doi: 10.1161/01.CIR.0000143233.87854.23. - DOI - PubMed
-
- Calcagno C, Cornily JC, Hyafil F, Rudd JH, Briley-Saebo KC, Mani V, Goldschlager G, Machac J, Fuster V, Fayad ZA. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–7. doi: 10.1161/ATVBAHA.108.166173. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

